TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization
- PMID: 20103683
- PMCID: PMC2824550
- DOI: 10.1158/1078-0432.CCR-09-2604
TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization
Abstract
Purpose: This study aims to investigate the role of the aberrant expression of Transkelolase-like 1 (TKTL1) in head and neck squamous cell carcinoma (HNSCC) tumorigenesis and to characterize TKTL1 contribution to HNSCC tumorigenesis through aerobic glycolysis and HIF1alpha stabilization.
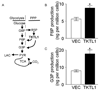
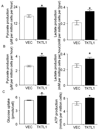
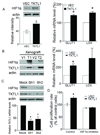
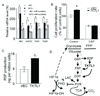
Experimental design: TKTL1 promoter hypomethylation and mRNA/protein aberrant expression were studied in human HNSCC tumor samples and normal mucosas. Oncogenic functions of TKTL1 were examined in HNSCC cell line panels and tumor xenograft models with TKTL1 expression construct. The metabolite levels of fructose-6-phosphate, glyceraldehydes-3-phosphate, pyruvate, lactate, and the levels of HIF1alpha protein and its downsteam glycolytic targets were compared between the TKTL1-expressing and vehicle-expressing HNSCC cells. Meanwhile, the effects of HIF1alpha/glycolytic inhibitors were evaluated on the TKTL1 transfectants.
Results: TKTL1 exhibits high frequency of promoter hypomethylation in HNSCC tumors compared with the normal mucosas, correlating with its overexpression in HNSCC. Overexpression of TKTL1 in HNSCC cells promoted cellular proliferation and enhanced tumor growth in vitro and in vivo. Overexpression of TKTL1 increased the production of fructose-6-phosphate and glyceraldehyde-3-phosphate, in turn elevating the production of pyruvate and lactate, resulting in the normoxic stabilization of the malignancy-promoting transcription factor HIF1alpha and the upregulation of downstream glycolytic enzymes. Notably, the reduction of TKTL1 expression decreased HIF1alpha accumulation and inhibition with HIF1alpha and/or the glycolysis inhibitor could abrogate the growth effects mediated by TKTL1 overexpression.
Conclusion: TKTL1 is a novel candidate oncogene that is epigenetically activated by aberrant hypomethlation and contributes to a malignant phenotype through altered glycolytic metabolism and HIF1alpha accumulation.
Figures


References
-
- Carvalho AL, Jeronimo C, Kim MM, et al. Evaluation of promoter hypermethylation detection in body fluids as a screening/diagnosis tool for head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:97–107. - PubMed
-
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. - PubMed
-
- Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–1828. - PubMed
-
- Nishigaki M, Aoyagi K, Danjoh I, et al. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res. 2005;65:2115–2124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

