Biological activity of celecoxib in the bronchial epithelium of current and former smokers
- PMID: 20103722
- PMCID: PMC4028718
- DOI: 10.1158/1940-6207.CAPR-09-0233
Biological activity of celecoxib in the bronchial epithelium of current and former smokers
Abstract
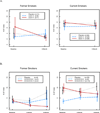
Non-small cell lung cancer is the primary cause of cancer-related death in Western countries. One important approach taken to address this problem is the development of effective chemoprevention strategies. In this study, we examined whether the cyclooxygenase-2 inhibitor celecoxib, as evidenced by decreased cell proliferation, is biologically active in the bronchial epithelium of current and former smokers. Current or former smokers with at least a 20 pack-year (pack-year = number of packs of cigarettes per day times number of years smoked) smoking history were randomized into one of four treatment arms (3-month intervals of celecoxib then placebo, celecoxib then celecoxib, placebo then celecoxib, or placebo then placebo) and underwent bronchoscopies with biopsies at baseline, 3 months, and 6 months. The 204 patients were primarily (79.4%) current smokers: 81 received either low-dose celecoxib or placebo and 123 received either high-dose celecoxib or placebo. Celecoxib was originally administered orally at 200 mg twice daily and the protocol subsequently increased the dose to 400 mg twice daily. The primary end point was change in Ki-67 labeling (from baseline to 3 months) in bronchial epithelium. No cardiac toxicities were observed in the participants. Although the effect of low-dose treatment was not significant, high-dose celecoxib decreased Ki-67 labeling by 3.85% in former smokers and by 1.10% in current smokers-a significantly greater reduction (P = 0.02) than that seen with placebo after adjusting for metaplasia and smoking status. A 3- to 6-month celecoxib regimen proved safe to administer. Celecoxib (400 mg twice daily) was biologically active in the bronchial epithelium of current and former smokers; additional studies on the efficacy of celecoxib in non-small cell lung cancer chemoprevention may be warranted.
Conflict of interest statement
The authors have disclosed no potential conflicts of interest.
Figures



Comment in
-
Assessing efficacy in early-phase cancer prevention clinical trials: the case of ki-67 in the lung.Cancer Prev Res (Phila). 2010 Feb;3(2):128-31. doi: 10.1158/1940-6207.CAPR-09-0268. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103726 Free PMC article. Review.
Similar articles
-
Assessing efficacy in early-phase cancer prevention clinical trials: the case of ki-67 in the lung.Cancer Prev Res (Phila). 2010 Feb;3(2):128-31. doi: 10.1158/1940-6207.CAPR-09-0268. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103726 Free PMC article. Review.
-
Lung cancer chemoprevention with celecoxib in former smokers.Cancer Prev Res (Phila). 2011 Jul;4(7):984-93. doi: 10.1158/1940-6207.CAPR-11-0078. Cancer Prev Res (Phila). 2011. PMID: 21733822 Free PMC article. Clinical Trial.
-
Celecoxib decreases Ki-67 proliferative index in active smokers.Clin Cancer Res. 2006 Jan 1;12(1):314-20. doi: 10.1158/1078-0432.CCR-05-1440. Clin Cancer Res. 2006. PMID: 16397057 Clinical Trial.
-
Proliferative changes in the bronchial epithelium of former smokers treated with retinoids.J Natl Cancer Inst. 2007 Nov 7;99(21):1603-12. doi: 10.1093/jnci/djm205. Epub 2007 Oct 30. J Natl Cancer Inst. 2007. PMID: 17971525 Free PMC article. Clinical Trial.
-
Long-term impact of smoking on lung epithelial proliferation in current and former smokers.J Natl Cancer Inst. 2001 Jul 18;93(14):1081-8. doi: 10.1093/jnci/93.14.1081. J Natl Cancer Inst. 2001. PMID: 11459869
Cited by
-
Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity.Biology (Basel). 2020 Dec 1;9(12):434. doi: 10.3390/biology9120434. Biology (Basel). 2020. PMID: 33271839 Free PMC article. Review.
-
A Pilot Study of a Grape Seed Procyanidin Extract for Lung Cancer Chemoprevention.Cancer Prev Res (Phila). 2019 Aug;12(8):557-566. doi: 10.1158/1940-6207.CAPR-19-0053. Epub 2019 May 28. Cancer Prev Res (Phila). 2019. PMID: 31138523 Free PMC article. Clinical Trial.
-
Phase II cancer prevention clinical trials.Semin Oncol. 2010 Aug;37(4):359-66. doi: 10.1053/j.seminoncol.2010.06.015. Semin Oncol. 2010. PMID: 20816506 Free PMC article. Review.
-
Assessing efficacy in early-phase cancer prevention clinical trials: the case of ki-67 in the lung.Cancer Prev Res (Phila). 2010 Feb;3(2):128-31. doi: 10.1158/1940-6207.CAPR-09-0268. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103726 Free PMC article. Review.
-
Oral iloprost improves endobronchial dysplasia in former smokers.Cancer Prev Res (Phila). 2011 Jun;4(6):793-802. doi: 10.1158/1940-6207.CAPR-11-0057. Cancer Prev Res (Phila). 2011. PMID: 21636546 Free PMC article. Clinical Trial.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- U.S. Department of Health and Human Services. The health benefits of smoking cessation: a report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 1990 DHHS Publication No. (CDC) 90-8416.
-
- Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of NSCLC mortality in former smokers. J Natl Cancer Inst. 1993;85:457–464. - PubMed
-
- Burns DM. Primary prevention, smoking, and smoking cessation: implications for future trends in NSCLC prevention. Cancer. 2000;89(11 Suppl):2506–2509. - PubMed
-
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on NSCLC risk. JAMA. 2005;294(12):1505–1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

