A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells
- PMID: 20103773
- PMCID: PMC2940714
- DOI: 10.1126/scisignal.2000514
A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells
Abstract
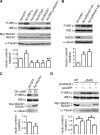
Autophosphorylation of inositol-requiring enzyme 1alpha (IRE1alpha) is required for its activation, which elicits the cellular unfolded protein response (UPR) and is functionally connected with insulin biosynthesis in pancreatic beta cells. We found that the scaffold protein receptor for activated C-kinase 1 (RACK1) interacted with IRE1alpha in a glucose-stimulated or endoplasmic reticulum (ER) stress-responsive manner in pancreatic beta cells and primary islets. RACK1 mediated the glucose-inducible assembly of a complex containing IRE1alpha, RACK1, and protein phosphatase 2A (PP2A) to promote dephosphorylation of IRE1alpha by PP2A, thereby inhibiting glucose-stimulated IRE1alpha activation and attenuating IRE1alpha-dependent increases in insulin production. Moreover, IRE1alpha activation was increased and RACK1 abundance was decreased in a mouse model of diabetes. Thus, our findings demonstrate that RACK1 functions as a key component in regulating the IRE1alpha signaling pathway in pancreatic beta cells.
Figures
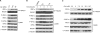

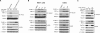
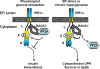
References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. - PubMed
-
- Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28− related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. - PubMed
-
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. - PubMed
-
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

