TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2
- PMID: 20103810
- PMCID: PMC3035503
- DOI: 10.1194/jlr.M002410
TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2
Abstract
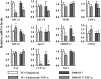
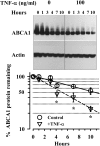
HDL cholesterol levels are decreased in Crohn's disease, a tumor necrosis factor-alpha (TNF-alpha)-driven chronic inflammatory condition involving the gastrointestinal tract. ATP-binding cassette transporter A1 (ABCA1), one of several liver X receptor (LXR) target genes, is a cell surface transporter that mediates the rate-controlling step in HDL synthesis. The regulation of ABCA1 and HDL cholesterol efflux by TNF-alpha was investigated in the human intestinal cell line Caco-2. In response to cholesterol micelles or T0901317, an LXR nonsterol agonist, TNF-alpha decreased the basolateral efflux of cholesterol to apolipoprotein A1 (apoA1). TNF-alpha, by attenuating ABCA1 promoter activity, markedly decreased ABCA1 gene expression without attenuating the expression of LXR-alpha, LXR-beta, and most other LXR target genes, such as ABCG1, FAS, ABCG8, scavenger receptor-B1 (SR-B1), and apoC1. TNF-alpha also decreased ABCA1 mass by markedly enhancing the rate of ABCA1 degradation and modestly inhibiting its rate of synthesis. Inhibitors of the nuclear factor-kappaB (NF-kappaB) pathway, which is activated by TNF-alpha, partially reverse the effect of TNF-alpha on ABCA1 protein expression. The results suggest that TNF-alpha, the major cytokine implicated in the inflammation of Crohn's disease, decreases HDL cholesterol levels by attenuating the expression of intestinal ABCA1 and cholesterol efflux to apoA1.
Figures





Similar articles
-
LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells.J Lipid Res. 2002 Jul;43(7):1054-64. doi: 10.1194/jlr.m100358-jlr200. J Lipid Res. 2002. PMID: 12091489
-
Liver-X-receptor-mediated increase in ATP-binding cassette transporter A1 expression is attenuated by fatty acids in CaCo-2 cells: effect on cholesterol efflux to high-density lipoprotein.Biochem J. 2004 Feb 1;377(Pt 3):545-52. doi: 10.1042/BJ20030903. Biochem J. 2004. PMID: 14604434 Free PMC article.
-
LXR driven induction of HDL-cholesterol is independent of intestinal cholesterol absorption and ABCA1 protein expression.Lipids. 2014 Jan;49(1):71-83. doi: 10.1007/s11745-013-3853-8. Epub 2013 Oct 27. Lipids. 2014. PMID: 24163219
-
ABCA1 and atherosclerosis.Vasc Med. 2005 May;10(2):109-19. doi: 10.1191/1358863x05vm593ra. Vasc Med. 2005. PMID: 16013195 Review.
-
ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis.Curr Drug Targets. 2011 May;12(5):647-60. doi: 10.2174/138945011795378522. Curr Drug Targets. 2011. PMID: 21039336 Review.
Cited by
-
TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells.Int J Mol Sci. 2021 Jun 9;22(12):6236. doi: 10.3390/ijms22126236. Int J Mol Sci. 2021. PMID: 34207810 Free PMC article.
-
Intestinal IL-25 prevents high-fat diet-induced obesity by modulating the cholesterol transporter NPC1L1 expression in the intestinal epithelial cells.Sci Rep. 2025 Mar 26;15(1):10445. doi: 10.1038/s41598-025-95516-7. Sci Rep. 2025. PMID: 40140439 Free PMC article.
-
MCP-1 impacts RCT by repressing ABCA1, ABCG1, and SR-BI through PI3K/Akt posttranslational regulation in HepG2 cells.J Lipid Res. 2013 May;54(5):1231-40. doi: 10.1194/jlr.M032482. Epub 2013 Feb 12. J Lipid Res. 2013. PMID: 23402987 Free PMC article.
-
Associations of Circulating Protein Levels With Lipid Fractions in the General Population.Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2505-2518. doi: 10.1161/ATVBAHA.118.311440. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354202 Free PMC article.
-
Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease.Mol Cell Biochem. 2018 Mar;440(1-2):167-187. doi: 10.1007/s11010-017-3165-z. Epub 2017 Aug 21. Mol Cell Biochem. 2018. PMID: 28828539 Review.
References
-
- Rhoads G. G., Gulbrandsen C. L., Kagan A. 1976. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N. Engl. J. Med. 294: 293–298. - PubMed
-
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714. - PubMed
-
- Sharrett A. R., Ballantyne C. M., Coady S. A., Heiss G., Sorlie P. D., Catellier D., Patsch W. 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 104: 1108–1113. - PubMed
-
- Wang N., Tall A. R. 2003. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 1178–1184. - PubMed
-
- Brewer H. B., Jr., Remaley A. T., Neufeld E. B., Basso F., Joyce C. 2004. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 24: 1755–1760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

