A transposon and transposase system for human application
- PMID: 20104209
- PMCID: PMC2862530
- DOI: 10.1038/mt.2010.2
A transposon and transposase system for human application
Abstract
The stable introduction of therapeutic transgenes into human cells can be accomplished using viral and nonviral approaches. Transduction with clinical-grade recombinant viruses offers the potential of efficient gene transfer into primary cells and has a record of therapeutic successes. However, widespread application for gene therapy using viruses can be limited by their initially high cost of manufacture at a limited number of production facilities as well as a propensity for nonrandom patterns of integration. The ex vivo application of transposon-mediated gene transfer now offers an alternative to the use of viral vectors. Clinical-grade DNA plasmids can be prepared at much reduced cost and with lower immunogenicity, and the integration efficiency can be improved by the transient coexpression of a hyperactive transposase. This has facilitated the design of human trials using the Sleeping Beauty (SB) transposon system to introduce a chimeric antigen receptor (CAR) to redirect the specificity of human T cells. This review examines the rationale and safety implications of application of the SB system to genetically modify T cells to be manufactured in compliance with current good manufacturing practice (cGMP) for phase I/II trials.
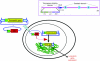
Figures


References
-
- Kay MA, Glorioso JC., and , Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. - PubMed
-
- Lozier JN, Csako G, Mondoro TH, Krizek DM, Metzger ME, Costello R, et al. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum Gene Ther. 2002;13:113–124. - PubMed
-
- Graham A, Walker R, Baird P, Hahn CN., and , Fazakerley JK. CNS gene therapy applications of the Semliki Forest virus 1 vector are limited by neurotoxicity. Mol Ther. 2006;13:631–635. - PubMed
-
- Henikoff S. Conspiracy of silence among repeated transgenes. Bioessays. 1998;20:532–535. - PubMed
-
- Selker EU. Gene silencing: repeats that count. Cell. 1999;97:157–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

