Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease
- PMID: 20104213
- PMCID: PMC2839425
- DOI: 10.1038/mt.2009.305
Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease
Abstract
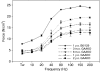
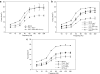
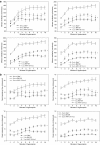
Pompe disease is a muscular dystrophy that results in respiratory insufficiency. We characterized the outcomes of targeted delivery of recombinant adeno-associated virus serotype 1 (rAAV2/1) vector to diaphragms of Pompe mice with varying stages of disease progression. We observed significant improvement in diaphragm contractile strength in mice treated at 3 months of age that is sustained at least for 1 year and enhanced contractile strength in mice treated at 9 and 21 months of age, measured 3 months post-treatment. Ventilatory parameters including tidal volume/inspiratory time ratio, minute ventilation/expired CO2 ratio, and peak inspiratory airflow were significantly improved in mice treated at 3 months and tested at 6 months. Despite early improvement, mice treated at 3 months and tested at 1 year had diminished normoxic ventilation, potentially due to attenuation of correction over time or progressive degeneration of nontargeted accessory tissues. However, for all rAAV2/1-treated mice (treated at 3, 9, and 21 months, assayed 3 months later; treated at 3 months, assayed at 1 year), minute ventilation and peak inspiratory flows were significantly improved during respiratory challenge. These results demonstrate that gel-mediated delivery of rAAV2/1 vectors can significantly augment ventilatory function at initial and late phases of disease in a model of muscular dystrophy.
Figures


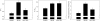
References
-
- Hirschhorn R., and , Reuser AJJ.2001Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiencyIn: Scriver CR, Beaudet A, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease McGraw-Hill: New York, pp 3389–3420
-
- Baudhuin P, Hers HG., and , Loeb H. An electron microscopic and biochemical study of type II glycogenosis. Lab Invest. 1964;13:1139–1152. - PubMed
-
- Raben N, Plotz P., and , Byrne BJ. Acid α-glucosidase deficiency (glycogenosis type II, Pompe disease. Curr Mol Med. 2002;2:145–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

