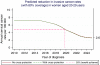
Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20-29 in the UK
- PMID: 20104226
- PMCID: PMC2833241
- DOI: 10.1038/sj.bjc.6605528
Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20-29 in the UK
Abstract
Background: Human papillomavirus (HPV) vaccination has been approved in more than 90 countries and is being implemented in many of these. In the UK, vaccination for girls aged 12-13 with catch-up for girls up to age 18 was introduced in 2008, using the bivalent GSK vaccine (Cervarix).
Methods: We modelled the proportion of abnormal smears, cervical intraepithelial neoplasia grade 3 (CIN3) and invasive cancer, which will be prevented in women aged 20-29 in the UK as a result of HPV vaccination.
Results: It will take many years for the full benefit of vaccination to be achieved. The earliest effects will be seen in women aged 20-29. With 80% coverage in women aged 12-13, we project an eventual 63% reduction in invasive cancer, a 51% reduction in CIN3 and a 27% reduction in cytological abnormalities before age 30. The full effect in this age group will not be seen until 2025, although half of the benefit will be seen by 2019 in England, where screening starts at age 25. However in Scotland and Wales, where screening starts at age 20, 50% of the benefit for CIN3 and abnormal smears (but not cancer) will be seen earlier.
Conclusion: Substantial reductions in disease can be anticipated by vaccination, but most of the benefit will not be apparent for at least another decade. High vaccine coverage is the key factor for achieving these benefits.
Figures
Similar articles
-
Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials.BMJ Open. 2017 Aug 18;7(8):e015867. doi: 10.1136/bmjopen-2017-015867. BMJ Open. 2017. PMID: 28821519 Free PMC article. Clinical Trial.
-
Cervical adenocarcinoma in situ: Human papillomavirus types and incidence trends in five states, 2008-2015.Int J Cancer. 2020 Feb 1;146(3):810-818. doi: 10.1002/ijc.32340. Epub 2019 May 6. Int J Cancer. 2020. PMID: 30980692 Free PMC article.
-
Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial.Lancet Oncol. 2012 Jan;13(1):89-99. doi: 10.1016/S1470-2045(11)70286-8. Epub 2011 Nov 8. Lancet Oncol. 2012. PMID: 22075171 Clinical Trial.
-
Human papillomavirus vaccines versus cervical cancer screening.Clin Oncol (R Coll Radiol). 2008 Aug;20(6):388-94. doi: 10.1016/j.clon.2008.04.006. Epub 2008 Jun 5. Clin Oncol (R Coll Radiol). 2008. PMID: 18538554 Review.
-
[Diseases caused by human papilloma viruses].Dtsch Med Wochenschr. 2011 May;136(20):1067-72. doi: 10.1055/s-0031-1275845. Epub 2011 May 10. Dtsch Med Wochenschr. 2011. PMID: 21560109 Review. German.
Cited by
-
Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States.Vaccine. 2013 Jun 24;31(29):3019-24. doi: 10.1016/j.vaccine.2013.04.051. Epub 2013 May 10. Vaccine. 2013. PMID: 23664991 Free PMC article.
-
Distribution of high and low risk HPV types by cytological status: a population based study from Italy.Infect Agent Cancer. 2011 Jan 20;6:2. doi: 10.1186/1750-9378-6-2. Infect Agent Cancer. 2011. PMID: 21247508 Free PMC article.
-
Willingness to human papillomavirus (HPV) vaccination and influencing factors among male and female university students in China.J Med Virol. 2022 Jun;94(6):2776-2786. doi: 10.1002/jmv.27478. Epub 2021 Dec 6. J Med Virol. 2022. PMID: 34825712 Free PMC article.
-
Worldwide cancer statistics of adolescents and young adults in 2019: a systematic analysis of the Global Burden of Disease Study 2019.ESMO Open. 2021 Oct;6(5):100255. doi: 10.1016/j.esmoop.2021.100255. Epub 2021 Sep 1. ESMO Open. 2021. PMID: 34481330 Free PMC article.
-
Long-term follow-up in cancer prevention trials (It ain't over 'til it's over).Cancer Prev Res (Phila). 2010 Jun;3(6):689-91. doi: 10.1158/1940-6207.CAPR-10-0096. Cancer Prev Res (Phila). 2010. PMID: 20522799 Free PMC article.
References
-
- Brotherton JM, Deeks SL, Campbell-Lloyd S, Misrachi A, Passaris I, Peterson K, Pitcher H, Scully M, Watson M, Webby R (2008) Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intel 32(4): 457–461 - PubMed
-
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Muñoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Sings HL, James M, Hesley TM, Barr E (2009) The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 199(7): 926–935 - PubMed
-
- Bruni L, Ferrer E, Diaz M, Louie KS, Albero G, Muñoz J, Castellsagué X, Bosch F, de Sanjosé S (2009) Worldwide HPV type-specific prevalence in cytologically normal women (1995–2008). Abstract O-30.05. 25th International Papilloma Virus Conference. Malmo, Sweden
-
- Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ (2007) Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370(9601): 1764–1772 - PubMed
-
- Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL (2006) Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24(Suppl 3): S3/26-34 - PubMed