Hypoxia inducible factors in cancer stem cells
- PMID: 20104230
- PMCID: PMC2833246
- DOI: 10.1038/sj.bjc.6605551
Hypoxia inducible factors in cancer stem cells
Abstract
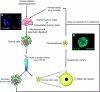
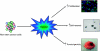
Oxygen is an essential regulator of cellular metabolism, survival, and proliferation. Cellular responses to oxygen levels are monitored, in part, by the transcriptional activity of the hypoxia inducible factors (HIFs). Under hypoxia, HIFs regulate a variety of pro-angiogenic and pro-glycolysis pathways. In solid cancers, regions of hypoxia are commonly present throughout the tissue because of the chaotic vascular architecture and regions of necrosis. In these regions, the hypoxic state fluctuates in a spatial and temporal manner. Transient hypoxic cycling causes an increase in the activity of the HIF proteins above what is typical for non-pathologic tissue. The extent of hypoxia strongly correlates to poor patient survival, therapeutic resistance and an aggressive tumour phenotype, but the full contribution of hypoxia and the HIFs to tumour biology is an area of active investigation. Recent reports link resistance to conventional therapies and the metastatic potential to a stem-like tumour population, termed cancer stem cells (CSCs). We and others have shown that within brain tumours CSCs reside in two niches, a perivascular location and the surrounding necrotic tissue. Restricted oxygen conditions increase the CSC fraction and promote acquisition of a stem-like state. Cancer stem cells are critically dependant on the HIFs for survival, self-renewal, and tumour growth. These observations and those from normal stem cell biology provide a new mechanistic explanation for the contribution of hypoxia to malignancy. Further, the presence of hypoxia in tumours may present challenges for therapy because of the promotion of CSC phenotypes even upon successful killing of CSCs. The current experimental evidence suggests that CSCs are plastic cell states governed by microenvironmental conditions, such as hypoxia, that may be critical for the development of new therapies targeted to disrupt the microenvironment.
Figures

References
-
- Baish JW, Jain RK (1998) Cancer, angiogenesis and fractals. Nat Med 4: 984 - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006a) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760 - PubMed
-
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006b) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66: 7843–7848 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

