In vitro assessment of platelet storage lesion in leucoreduced random donor platelet concentrates
- PMID: 20104276
- PMCID: PMC2809509
- DOI: 10.2450/2009.0077-09
In vitro assessment of platelet storage lesion in leucoreduced random donor platelet concentrates
Abstract
Background: Currently platelet concentrates (PC) are collected using different synthetic materials and different centrifugation/leucocyte-removal processes. Upon exposure to artificial surfaces and high centrifugation forces, blood cells can undergo various levels of stress-induced, cellular activation/fragmentation and release reactions which may not only influence the extent of the platelet storage lesion but may also contribute to poor clinical effectiveness of the PC and transfusion reactions.
Materials and methods: An array of assays, used for quality control of PC, was performed in two different groups of PC prepared from random donor plasma on days 1, 3 and 5 of storage. The group 1 PC were not leucoreduced while the group 2 PC underwent prestorage leucoreduction using a PL50E filter. As current recommendations for the evaluation of PC include the measurement of platelet activation, in this study CD62P on platelet membrane was measured. Furthermore, in vitro studies indicate that sHLA antigens may modulate immune competent cell function so, the presence of sHLA-1 in blood components is considered a marker of immunological reactivity and this, too, was measured.
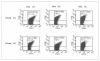
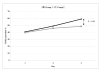
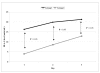
Results: The levels of CD62P and sHLA-1 were significantly lower in leucoreduced PC than in non-leucoreduced ones. However, the overall rate of increase of sHLA-1 during storage was faster in the leucoreduced group of PC. No significant differences were detected regarding other assays of quality.
Conclusion: Based on our findings, leucoreduced PC differ from non-leucoreduced ones in terms of some specific markers such as CD62P as a marker of platelet activation and sHLA-1 as a marker of immunological reactivity. Pre-storage leucofiltration, followed by storage in currently used plastic bags is a safe procedure for PC for up to 5 days. The available leucoreduction technologies are not, however, sufficiently robust to completely abrogate transfusions reactions, and improvements are required to reach the goal of optimised yield and minimal transfusion reactions with platelet therapy.
Keywords: CD62P; platelet concentrate; platelet storage lesion; sHLA-1.
Figures
References
-
- Wallace EL, Churchill WH, Surgenor DM, Cho GS. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998;38:625–36. - PubMed
-
- Klinger MH. The storage lesion of ultrastructural and functional aspects. Ann Hematol. 1996;73:103–12. - PubMed
-
- Seghatchian J, Krailadsiri P.Platelet storage lesion and apoptosis: are they related? Transfus Apheresis Sci 200124103–5.[Review] - PubMed
-
- Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfus Med Rev. 1997;11:130–44. - PubMed
-
- Krailadsiri P, Seghatchian J, Williamson LM. Platelet storage lesion of WBC-reduced pooled buffy coat-derived PC prepared in three in-process filtered/storage bag combinations. Transfusion. 2001;41:243–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
