Comparison of natural histories of human papillomavirus detected by clinician- and self-sampling
- PMID: 20104517
- PMCID: PMC3132413
- DOI: 10.1002/ijc.25199
Comparison of natural histories of human papillomavirus detected by clinician- and self-sampling
Abstract
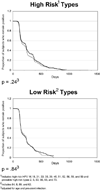
New strategies for cervical cancer screening include human papillomavirus (HPV) DNA testing. Using self-testing methods would increase access to testing in both developed and developing countries. The purpose of this study was to compare time-to-clearance of specific HPV types between clinician-collected-lavage (CC-L) and self-collected (SC) sampling in a single cohort. CC-L and SC samples were obtained every 4 months at alternate 2-month windows from 537 women. Eighteen high-risk (HR) HPV and 4 low-risk (LR) HPV were examined. Proportional hazards model was used to compare time-to-clearance between methods for combined HR and for 13 specific HPV types. Prentice-Wilcoxon test was used for within-subject paired comparison. In the independent analysis for combined HR and LR types, no differences were found. For specific types, time-to-clearance for all HPV types examined between CC-L and SC samples was similar except for HPV 66 which showed a trend to clear slower by SC (p = 0.09). When comparing methods in the same woman, time-to-clearance was similar for all types except for HPV 16 which showed a trend to clear slower by CC-L means (p = 0.08). When we examined pattern of clearance among the CC-L samples, the fastest types to clear were HPV 6, 18, 66, 84 and 39 and the slowest were HPV 62, 68, 59 and 16. These patterns of fast and slow were similar for SC samples. Our findings suggest using SC vaginal swabs would observe similar natural histories of HPV compared to studies using CC-L specimens making self-testing feasible for repeated HPV DNA detection.
Figures



Similar articles
-
Self-collected vaginal sampling for the detection of genital human papillomavirus (HPV) using careHPV among Ghanaian women.BMC Womens Health. 2017 Sep 26;17(1):86. doi: 10.1186/s12905-017-0448-1. BMC Womens Health. 2017. PMID: 28950841 Free PMC article.
-
Can self vaginal douching for high risk HPV screening replace or assist efficacy of cervical cancer screening?Asian Pac J Cancer Prev. 2010;11(5):1397-401. Asian Pac J Cancer Prev. 2010. PMID: 21198300
-
Interest of human papillomavirus DNA quantification and genotyping in paired cervical and urine samples to detect cervical lesions.Arch Gynecol Obstet. 2014 Aug;290(2):299-308. doi: 10.1007/s00404-014-3191-y. Epub 2014 Mar 13. Arch Gynecol Obstet. 2014. PMID: 24622934
-
Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction.Cancer Epidemiol Biomarkers Prev. 2001 Feb;10(2):95-100. Cancer Epidemiol Biomarkers Prev. 2001. PMID: 11219778
-
Self-obtained vaginal samples for HPV DNA testing to detect HPV-related cervical disease.Int J Gynaecol Obstet. 2021 Jul;154(1):127-132. doi: 10.1002/ijgo.13574. Epub 2021 Jan 27. Int J Gynaecol Obstet. 2021. PMID: 33368281
Cited by
-
Cervical-Vaginal Microbiome and Associated Cytokine Profiles in a Prospective Study of HPV 16 Acquisition, Persistence, and Clearance.Front Cell Infect Microbiol. 2020 Sep 25;10:569022. doi: 10.3389/fcimb.2020.569022. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33102255 Free PMC article.
-
Population-based type-specific prevalence of high-risk human papillomavirus infection in Estonia.BMC Infect Dis. 2010 Mar 11;10:63. doi: 10.1186/1471-2334-10-63. BMC Infect Dis. 2010. PMID: 20222944 Free PMC article.
-
Are vaginal swabs comparable to cervical smears for human papillomavirus DNA testing?J Gynecol Oncol. 2018 Jan;29(1):e8. doi: 10.3802/jgo.2018.29.e8. J Gynecol Oncol. 2018. PMID: 29185266 Free PMC article.
-
Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis.Can J Public Health. 2013 Feb 11;104(2):e159-66. doi: 10.1007/BF03405681. Can J Public Health. 2013. PMID: 23618210 Free PMC article.
-
Incidence and duration of type-specific human papillomavirus infection in high-risk HPV-naïve women: results from the control arm of a phase II HPV-16/18 vaccine trial.BMJ Open. 2016 Aug 26;6(8):e011371. doi: 10.1136/bmjopen-2016-011371. BMJ Open. 2016. PMID: 27566633 Free PMC article. Clinical Trial.
References
-
- Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. - PubMed
-
- Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, Darragh T, Palefsky J. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. - PubMed
-
- Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–738. - PubMed
-
- Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. - PubMed
-
- Agorastos T, Dinas K, Lloveras B, Font R, Kornegay JR, Bontis J, de Sanjose S. Self-sampling versus physician-sampling for human papillomavirus testing. Int J STD AIDS. 2005;16:727–729. - PubMed

