Genesis of teratogen-induced holoprosencephaly in mice
- PMID: 20104601
- PMCID: PMC2914459
- DOI: 10.1002/ajmg.c.30239
Genesis of teratogen-induced holoprosencephaly in mice
Abstract
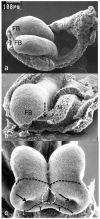
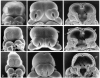
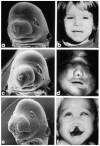
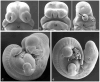
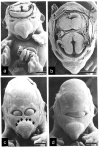
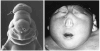
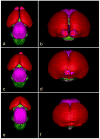
Evidence from mechanical, teratological, and genetic experimentation demonstrates that holoprosencephaly (HPE) typically results from insult prior to the time that neural tube closure is completed and occurs as a consequence of direct or indirect insult to the rostral prechordal cells that induce the forebrain or insult to the median forebrain tissue, itself. Here, we provide an overview of normal embryonic morphogenesis during the critical window for HPE induction, focusing on the morphology and positional relationship of the developing brain and subjacent prechordal plate and prechordal mesoderm cell populations. Subsequent morphogenesis of the HPE spectrum is then examined in selected teratogenesis mouse models. The temporal profile of Sonic Hedgehog expression in rostral embryonic cell populations and evidence for direct or indirect perturbation of the Hedgehog pathway by teratogenic agents in the genesis of HPE is highlighted. Emerging opportunities based on recent insights and new techniques to further characterize the mechanisms and pathogenesis of HPE are discussed.
2010 Wiley-Liss, Inc.
Figures







References
-
- Adelman H. The embryological basis of cyclopia. American Journal of Opthalmology. 1934;17:890–891.
-
- Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, Shioda S, Yamada G, Motoyama J. Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev Biol. 2009;327:106–20. - PubMed
-
- Blader P, Strahle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev Biol. 1998;201:185–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

