Comparative proteomics of human embryonic stem cells and embryonal carcinoma cells
- PMID: 20104618
- PMCID: PMC3086450
- DOI: 10.1002/pmic.200900483
Comparative proteomics of human embryonic stem cells and embryonal carcinoma cells
Abstract
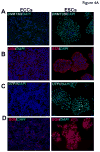
Pluripotent human embryonic stem cells (ESCs) can be differentiated in vitro into a variety of cells which hold promise for transplantation therapy. Human embryonal carcinoma cells (ECCs), stem cells of human teratocarcinomas, are considered a close but malignant counterpart to human ESCs. In this study, a comprehensive quantitative proteomic analysis of ESCs and ECCs was carried out using the iTRAQ method. Using two-dimensional LC and MS/MS analyses, we identified and quantitated approximately 1800 proteins. Among these are proteins associated with pluripotency and development as well as tight junction signaling and TGFbeta receptor pathway. Nearly approximately 200 proteins exhibit more than twofold difference in abundance between ESCs and ECCs. Examples of early developmental markers high in ESCs include beta-galactoside-binding lectin, undifferentiated embryonic cell transcription factor-1, DNA cytosine methyltransferase 3beta isoform-B, melanoma antigen family-A4, and interferon-induced transmembrane protein-1. In contrast, CD99-antigen (CD99), growth differentiation factor-3, cellular retinoic acid binding protein-2, and developmental pluripotency associated-4 were among the highly expressed proteins in ECCs. Several proteins that were highly expressed in ECCs such as heat shock 27 kDa protein-1, mitogen-activated protein kinase kinase-1, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor like-2, and S100 calcium-binding protein-A4 have also been attributed to malignancy in other systems. Importantly, immunocytochemistry was used to validate the proteomic analyses for a subset of the proteins. In summary, this is the first large-scale quantitative proteomic study of human ESCs and ECCs, which provides critical information about the regulators of these two closely related, but developmentally distinct, stem cells.
Figures





Similar articles
-
Plasma membrane proteomics of human embryonic stem cells and human embryonal carcinoma cells.J Proteome Res. 2008 Jul;7(7):2936-51. doi: 10.1021/pr800056j. Epub 2008 May 20. J Proteome Res. 2008. PMID: 18489135
-
iTRAQ proteome analysis reflects a progressed differentiation state of epiblast derived versus inner cell mass derived murine embryonic stem cells.J Proteomics. 2013 Sep 2;90:38-51. doi: 10.1016/j.jprot.2013.03.015. Epub 2013 Apr 18. J Proteomics. 2013. PMID: 23603003
-
Transforming pluripotency: an exon-level study of malignancy-specific transcripts in human embryonal carcinoma and embryonic stem cells.Stem Cells Dev. 2013 Apr 1;22(7):1136-46. doi: 10.1089/scd.2012.0369. Epub 2013 Jan 4. Stem Cells Dev. 2013. PMID: 23137282
-
Phosphoproteomic analysis: an emerging role in deciphering cellular signaling in human embryonic stem cells and their differentiated derivatives.Stem Cell Rev Rep. 2012 Mar;8(1):16-31. doi: 10.1007/s12015-011-9317-8. Stem Cell Rev Rep. 2012. PMID: 22009073 Free PMC article. Review.
-
A practical guide for the identification of membrane and plasma membrane proteins in human embryonic stem cells and human embryonal carcinoma cells.Proteomics. 2008 Oct;8(19):4036-53. doi: 10.1002/pmic.200800143. Proteomics. 2008. PMID: 18763709 Review.
Cited by
-
Systems Biology Approaches Applied to Regenerative Medicine.Curr Pathobiol Rep. 2015;3(1):37-45. doi: 10.1007/s40139-015-0072-4. Curr Pathobiol Rep. 2015. PMID: 25722955 Free PMC article. Review.
-
Two Effective Routes for Removing Lineage Restriction Roadblocks: From Somatic Cells to Hepatocytes.Int J Mol Sci. 2015 Sep 1;16(9):20873-95. doi: 10.3390/ijms160920873. Int J Mol Sci. 2015. PMID: 26340624 Free PMC article. Review.
-
TRA-1-60+, SSEA-4+, Oct4A+, Nanog+ Clones of Pluripotent Stem Cells in the Embryonal Carcinomas of the Ovaries.J Stem Cell Res Ther. 2012 Nov 18;2(5):130. J Stem Cell Res Ther. 2012. PMID: 23293749 Free PMC article.
-
Patterning Pluripotent Stem Cells at a Single Cell Level.J Biomater Tissue Eng. 2013 Aug;3(4):461-471. doi: 10.1166/jbt.2013.1106. Epub 2013 Aug 1. J Biomater Tissue Eng. 2013. PMID: 30135745 Free PMC article.
-
ERBB3-Binding Protein 1 (EBP1) Is a Novel Developmental Pluripotency-Associated-4 (DPPA4) Cofactor in Human Pluripotent Cells.Stem Cells. 2018 May;36(5):671-682. doi: 10.1002/stem.2776. Epub 2018 Jan 29. Stem Cells. 2018. PMID: 29327467 Free PMC article.
References
-
- Gearhart J. New potential for human embryonic stem cells. Science. 1998;282:1061–1062. - PubMed
-
- Rossant J. Stem cells: the magic brew. Nature. 2007;448:260–262. - PubMed
-
- Andrews PW, Damjanov I, Berends J, Kumpf S, et al. Inhibition of proliferation and induction of differentiation of pluripotent human embryonal carcinoma cells by osteogenic protein-1 (or bone morphogenetic protein-7) Lab Invest. 1994;71:243–251. - PubMed
-
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

