Several novel N-donor tridentate ligands formed in chemical studies of new fac-Re(CO)3 complexes relevant to fac-99mTc(CO)3 radiopharmaceuticals: attack of a terminal amine on coordinated acetonitrile
- PMID: 20104873
- PMCID: PMC2845929
- DOI: 10.1021/ic901705x
Several novel N-donor tridentate ligands formed in chemical studies of new fac-Re(CO)3 complexes relevant to fac-99mTc(CO)3 radiopharmaceuticals: attack of a terminal amine on coordinated acetonitrile
Abstract
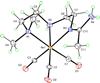
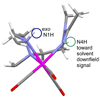
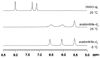
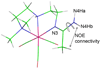
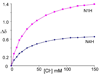
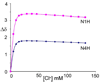
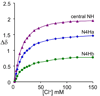
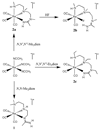
To evaluate syntheses of fac-[Re(CO)(3)L](+) complexes in organic solvents, we treated fac-[Re(CO)(3)(CH(3)CN)(3)]PF(6)/BF(4) in acetonitrile with triamine ligands (L). When L had two primary or two tertiary terminal amine groups, the expected fac-[Re(CO)(3)L](+) complexes formed. In contrast, N,N-dimethyldiethylenetriamine (N,N-Me(2)dien) formed an unusual compound, fac-[Re(CO)(3)(DAE)]BF(4) {DAE = (Z)-N'-(2-(2-(dimethylamino)ethylamino)ethyl)acetimidamide = (Me(2)NCH(2)CH(2))NH(CH(2)CH(2)N=C(NH(2))Me)}. DAE is formed by addition of acetonitrile to the N,N-Me(2)dien terminal primary amine, converting this sp(3) nitrogen to an sp(2) nitrogen with a double bond to the original acetonitrile sp carbon. The three Ns bound to Re derive from N,N-Me(2)dien. The pathway to fac-[Re(CO)(3)(DAE)]BF(4) is suggested by a second unusual compound, fac-[Re(CO)(3)(MAE)]PF(6) {MAE = N-methyl-N-(2-(methyl-(2-(methylamino)ethyl)amino)ethyl)acetimidamide = MeN(H)-CH(2)CH(2)-N(Me)-CH(2)CH(2)-N(Me)-C(Me)=NH}, isolated after treating fac-[Re(CO)(3)(CH(3)CN)(3)]PF(6) with N,N',N''-trimethyldiethylenetriamine (N,N',N''-Me(3)dien). MAE chelates via a terminal and a central sp(3) N from N,N',N''-Me(3)dien and via one sp(2) NH in a C(Me)=NH group. This group is derived from acetonitrile by addition of the other N,N',N''-Me(3)dien terminal amine to the nitrile carbon. This addition creates an endocyclic NMe group within a seven-membered chelate ring. The structure and other properties of fac-[Re(CO)(3)(MAE)]PF(6) allow us to propose a reaction scheme for the formation of the unprecedented DAE ligand. The new compounds advance our understanding of the spectral and structural properties of Re analogues of (99m)Tc radiopharmaceuticals.
Figures
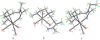

Similar articles
-
fac-[Re(CO)(3)L](+) complexes with N-CH(2)-CH(2)-X-CH(2)-CH(2)-N tridentate ligands. synthetic, X-ray crystallographic, and NMR spectroscopic investigations.Inorg Chem. 2007 Dec 24;46(26):11173-82. doi: 10.1021/ic701576u. Epub 2007 Nov 29. Inorg Chem. 2007. PMID: 18044880
-
fac-Re(CO)3L complexes containing tridentate monoanionic ligands (L-) with a seldom-studied sulfonamido group as one terminal ligating group.Inorg Chem. 2007 Aug 20;46(17):6942-9. doi: 10.1021/ic700594a. Epub 2007 Jul 26. Inorg Chem. 2007. PMID: 17655222
-
Complexes possessing rare "tertiary" sulfonamide nitrogen-to-metal bonds of normal length: fac-[Re(CO)3(N(SO2R)dien)]PF6 complexes with hydrophilic sulfonamide ligands.Inorg Chem. 2014 Jan 21;53(2):1144-55. doi: 10.1021/ic4026987. Epub 2014 Jan 8. Inorg Chem. 2014. PMID: 24400928 Free PMC article.
-
New monodentate amidine superbasic ligands with a single configuration in fac-[Re(CO)3(5,5'- or 6,6'-Me2bipyridine)(amidine)]BF4 complexes.Inorg Chem. 2012 Jul 2;51(13):7271-83. doi: 10.1021/ic300625n. Epub 2012 Jun 12. Inorg Chem. 2012. PMID: 22691073 Free PMC article.
-
Superbasic amidine monodentate ligands in fac-[Re(CO)3(5,5'-Me2bipy)(amidine)]BF4 complexes: dependence of amidine configuration on the remote nitrogen substituents.Inorg Chem. 2010 Aug 2;49(15):7035-45. doi: 10.1021/ic100714m. Inorg Chem. 2010. PMID: 20593850
Cited by
-
Re(CO)3-Templated Synthesis of α-Amidinoazadi(benzopyrro)methenes.Inorg Chem. 2017 Dec 18;56(24):14734-14737. doi: 10.1021/acs.inorgchem.7b02140. Epub 2017 Nov 27. Inorg Chem. 2017. PMID: 29172475 Free PMC article.
-
Structure and Properties of fac-[Re(I)(CO)3(NTA)](2-) (NTA(3-) = Trianion of Nitrilotriacetic Acid) and fac-[Re(I)(CO)3(L)](n-) Analogues Useful for Assessing the Excellent Renal Clearance of the fac-[(99m)Tc(I)(CO)3(NTA)](2-) Diagnostic Renal Agent.Inorg Chem. 2015 Jul 6;54(13):6281-90. doi: 10.1021/acs.inorgchem.5b00584. Epub 2015 Jun 12. Inorg Chem. 2015. PMID: 26068141 Free PMC article.
-
Design and synthesis of a bombesin peptide-conjugated tripodal phosphino dithioether ligand topology for the stabilization of the fac-[M(CO)3]+ core (M=(99 m)Tc or Re).Inorg Chem. 2011 Jul 4;50(13):6210-9. doi: 10.1021/ic200491z. Epub 2011 May 18. Inorg Chem. 2011. PMID: 21591746 Free PMC article.
-
NH NMR shifts of new structurally characterized fac-[Re(CO)3(polyamine)]n+ complexes probed via outer-sphere hydrogen-bonding interactions to anions, including the paramagnetic [Re(IV)Br6]2- anion.Inorg Chem. 2010 Jun 21;49(12):5560-72. doi: 10.1021/ic100386c. Inorg Chem. 2010. PMID: 20481637 Free PMC article.
References
-
- Alberto R, Schibli R, Schubiger AP, Abram U, Pietzsch HJ, Johannsen B. J. Am. Chem. Soc. 1999;121:6076–6077.
-
- Desbouis D, Struthers H, Spiwok V, Küster T, Schibli R. J. Med. Chem. 2008;51:6689–6698. - PubMed
-
- Wei L, Babich J, Zubieta J. Inorg. Chim. Acta. 2005;358:3691–3700.
-
- Schibli R, Schubiger AP. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1529–1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

