A novel non-natural nucleoside that influences P-glycoprotein activity and mediates drug resistance
- PMID: 20104904
- PMCID: PMC3164776
- DOI: 10.1021/bi9020428
A novel non-natural nucleoside that influences P-glycoprotein activity and mediates drug resistance
Abstract
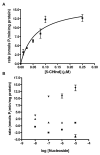
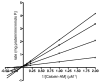
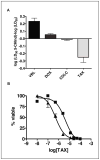
Multidrug resistance during cancer chemotherapy is commonly acquired by overexpression of the ATP binding cassette transporter, P-glycoprotein (P-gp). As such, inhibitors that target P-gp activity represent potential therapeutic agents against this form of drug resistance. This study evaluated the ability of various non-natural nucleosides that mimic the core structure of adenosine to modulate drug resistance by inhibiting the ATPase activity to P-gp. Of several analogues tested, only one novel non-natural nucleoside, 5-cyclohexylindolyl-2'-deoxyribose (5-CHInd), behaves as a P-gp inhibitor. Although 5-CHInd is an adenosine analogue that should block the binding of ATP, the non-natural nucleoside surprisingly stimulates the ATPase activity of P-gp in vitro. However, 5-CHInd is not an exportable substrate for P-gp as it is not transported across an MDCK-MDR1 monolayer. In addition, 5-CHInd differentially modulates MDR by decreasing or increasing the cytotoxicity of several chemotherapeutic agents. Although 5-CHInd displays variable activity in modulating the efflux of various drugs by P-gp, there is a correlation between changes observed in the drug-stimulated ATPase catalytic efficiency induced by 5-CHInd and its effect on drug efflux. The paradoxical behavior of 5-CHInd is rationalized within the context of contemporary models of P-gp function. In addition, the data are used to develop a predictive in vitro model for rapidly identifying potential drug-drug interactions with P-gp.
Figures
Similar articles
-
Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-glycoprotein.Molecules. 2020 Jan 7;25(2):247. doi: 10.3390/molecules25020247. Molecules. 2020. PMID: 31936160 Free PMC article.
-
Cinnamophilin overcomes cancer multi-drug resistance via allosterically modulating human P-glycoprotein on both drug binding sites and ATPase binding sites.Biomed Pharmacother. 2021 Dec;144:112379. doi: 10.1016/j.biopha.2021.112379. Epub 2021 Oct 28. Biomed Pharmacother. 2021. PMID: 34794239
-
β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function.Phytomedicine. 2016 Mar 15;23(3):316-23. doi: 10.1016/j.phymed.2016.01.008. Epub 2016 Feb 6. Phytomedicine. 2016. PMID: 26969385
-
Pharmacological strategies for overcoming multidrug resistance.Curr Drug Targets. 2006 Jul;7(7):861-79. doi: 10.2174/138945006777709593. Curr Drug Targets. 2006. PMID: 16842217 Review.
-
Discovery and biological evaluation of hederagenin derivatives as non-substrate inhibitors of P-glycoprotein-mediated multidrug resistance.Eur J Med Chem. 2025 May 5;289:117428. doi: 10.1016/j.ejmech.2025.117428. Epub 2025 Feb 20. Eur J Med Chem. 2025. PMID: 40010272 Review.
Cited by
-
Disruption of small molecule transporter systems by Transporter-Interfering Chemicals (TICs).FEBS Lett. 2020 Dec;594(23):4158-4185. doi: 10.1002/1873-3468.14005. Epub 2020 Dec 9. FEBS Lett. 2020. PMID: 33222203 Free PMC article. Review.
References
-
- Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. - PubMed
-
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. - PubMed
-
- Ling V, Kartner N, Sudo T, Siminovitch L, Riordan JR. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983;67:869–874. - PubMed
-
- Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009;109:2989–3011. - PubMed
-
- Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Jr, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

