Tetrandrine blocks cardiac hypertrophy by disrupting reactive oxygen species-dependent ERK1/2 signalling
- PMID: 20105174
- PMCID: PMC2829222
- DOI: 10.1111/j.1476-5381.2009.00605.x
Tetrandrine blocks cardiac hypertrophy by disrupting reactive oxygen species-dependent ERK1/2 signalling
Abstract
Background and purpose: Tetrandrine, a well-known naturally occurring calcium antagonist with anti-inflammatory, antioxidant and anti-fibrogenetic activities, has long been used clinically for treatment of cardiovascular diseases such as hypertension and arrhythmia. However, little is known about the effect of tetrandrine on cardiac hypertrophy. The aims of the present study were to determine whether tetrandrine could attenuate cardiac hypertrophy and to clarify the underlying molecular mechanisms.
Experimental approach: Tetrandrine (50 mg x kg(-1) x day(-1)) was administered by oral gavage three times a day for one week and then the mice were subjected to either chronic pressure overload generated by aortic banding (AB) or sham surgery (control group). Cardiac function was determined by echocardiography.
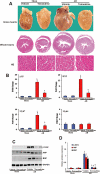
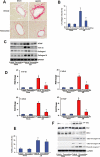
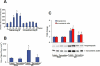
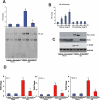
Key results: Tetrandrine attenuated the cardiac hypertrophy induced by AB, as assessed by heart weight/body weight and lung weight/body weight ratios, cardiac dilatation and the expression of genes of hypertrophic markers. Tetrandrine also inhibited fibrosis and attenuated the inflammatory response. The cardioprotective effects of tetrandrine were mediated by blocking the increased production of reactive oxygen species and the activation of ERK1/2-dependent nuclear factor-kappaB and nuclear factor of activated T cells that occur in response to hypertrophic stimuli.
Conclusions and implications: Taken together, our results suggest that tetrandrine can improve cardiac function and prevent the development of cardiac hypertrophy by suppressing the reactive oxygen species-dependent ERK1/2 signalling pathway.
Figures

Similar articles
-
Puerarin inhibits angiotensin II-induced cardiac hypertrophy via the redox-sensitive ERK1/2, p38 and NF-κB pathways.Acta Pharmacol Sin. 2014 Apr;35(4):463-75. doi: 10.1038/aps.2013.185. Epub 2014 Mar 10. Acta Pharmacol Sin. 2014. PMID: 24608673 Free PMC article.
-
Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway.J Cell Mol Med. 2009 May;13(5):909-25. doi: 10.1111/j.1582-4934.2008.00620.x. Epub 2008 Dec 24. J Cell Mol Med. 2009. PMID: 19413885 Free PMC article.
-
Ginseng reverses established cardiomyocyte hypertrophy and postmyocardial infarction-induced hypertrophy and heart failure.Circ Heart Fail. 2012 Jul 1;5(4):504-14. doi: 10.1161/CIRCHEARTFAILURE.112.967489. Epub 2012 May 10. Circ Heart Fail. 2012. PMID: 22576957
-
Molecular mechanisms and therapeutic implications of tetrandrine and cepharanthine in T cell acute lymphoblastic leukemia and autoimmune diseases.Pharmacol Ther. 2021 Jan;217:107659. doi: 10.1016/j.pharmthera.2020.107659. Epub 2020 Aug 12. Pharmacol Ther. 2021. PMID: 32800789 Review.
-
Regression of pathological cardiac hypertrophy: signaling pathways and therapeutic targets.Pharmacol Ther. 2012 Sep;135(3):337-54. doi: 10.1016/j.pharmthera.2012.06.006. Epub 2012 Jun 29. Pharmacol Ther. 2012. PMID: 22750195 Free PMC article. Review.
Cited by
-
Puerarin inhibits angiotensin II-induced cardiac hypertrophy via the redox-sensitive ERK1/2, p38 and NF-κB pathways.Acta Pharmacol Sin. 2014 Apr;35(4):463-75. doi: 10.1038/aps.2013.185. Epub 2014 Mar 10. Acta Pharmacol Sin. 2014. PMID: 24608673 Free PMC article.
-
Molecular pathogenesis of myocardial remodeling and new potential therapeutic targets in chronic heart failure.Ital J Pediatr. 2012 Sep 12;38:41. doi: 10.1186/1824-7288-38-41. Ital J Pediatr. 2012. PMID: 22971785 Free PMC article. Review.
-
Tetrandrine Ameliorates Myocardial Ischemia Reperfusion Injury through miR-202-5p/TRPV2.Biomed Res Int. 2021 Mar 8;2021:8870674. doi: 10.1155/2021/8870674. eCollection 2021. Biomed Res Int. 2021. PMID: 33763489 Free PMC article.
-
Gastrodin protects against cardiac hypertrophy and fibrosis.Mol Cell Biochem. 2012 Jan;359(1-2):9-16. doi: 10.1007/s11010-011-0992-1. Epub 2011 Jul 16. Mol Cell Biochem. 2012. PMID: 21833534
-
Genistein attenuates pathological cardiac hypertrophy in vivo and in vitro.Herz. 2019 May;44(3):247-256. doi: 10.1007/s00059-017-4635-5. Epub 2017 Nov 3. Herz. 2019. PMID: 29101624 English.
References
-
- Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. - PubMed
-
- Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. - PubMed
-
- Barbato R, Menabò R, Dainese P, Carafoli E, Schiaffino S, Di Lisa F. Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res. 1996;78:821–828. - PubMed
-
- Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–2039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

