Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A1 receptor ligands
- PMID: 20105183
- PMCID: PMC2829203
- DOI: 10.1111/j.1476-5381.2009.00488.x
Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A1 receptor ligands
Abstract
Background and purpose: The introduction of fluorescence-based techniques, and in particular the development of fluorescent ligands, has allowed the study of G protein-coupled receptor pharmacology at the single cell and single molecule level. This study evaluated how the physicochemical nature of the linker and the fluorophore affected the pharmacological properties of fluorescent agonists and antagonists.
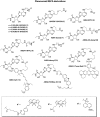
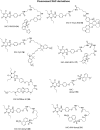
Experimental approach: Chinese hamster ovary cells stably expressing the human adenosine A(1) receptor and a cyclic 3',5' adenosine monophosphate response element-secreted placental alkaline phosphatase (CRE-SPAP) reporter gene, together with whole cell [(3)H]-8-cyclopentyl-1,3-dipropylxanthine (DPCPX) radioligand binding, were used to evaluate the pharmacological properties of a range of fluorescent ligands based on the antagonist xanthine amine congener (XAC) and the agonist 5' (N-ethylcarboxamido) adenosine (NECA).
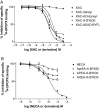
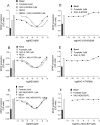
Key results: Derivatives of NECA and XAC with different fluorophores, but equivalent linker length, showed significant differences in their binding properties to the adenosine A(1) receptor. The BODIPY 630/650 derivatives had the highest affinity. Linker length also affected the pharmacological properties, depending on the fluorophore used. Particularly in fluorescent agonists, higher agonist potency could be achieved with large or small linkers for dansyl and BODIPY 630/650 derivatives, respectively.
Conclusions and implications: The pharmacology of a fluorescent ligand was critically influenced by both the fluorophore and the associated linker. Furthermore, our data strongly suggest that the physicochemical properties of the fluorophore/linker pairing determine where in the environment of the target receptor the fluorophore is placed, and this, together with the environmental sensitivity of the resulting fluorescence, may finally decide its utility as a fluorescent probe.
Figures




References
-
- Baker JG, Hill SJ. A comparison of the antagonist affinities for the Gi and Gs-coupled states of the human adenosine A1 receptor. J Pharmacol Exp Ther. 2007;320:218–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

