Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets
- PMID: 20105230
- PMCID: PMC2875871
- DOI: 10.1111/j.1460-9568.2010.07085.x
Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets
Abstract
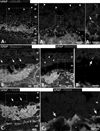
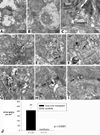
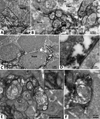
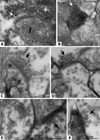
The aim of this study was to determine whether retinal progenitor layer transplants form synaptic connections with the host and restore vision. Donor retinal sheets, isolated from embryonic day 19 rat fetuses expressing human placental alkaline phosphatase (hPAP), were transplanted to the subretinal space of 18 S334ter-3 rats with fast retinal degeneration at the age of 0.8-1.3 months. Recipients were killed at the age of 1.6-11.8 months. Frozen sections were analysed by confocal immunohistochemistry for the donor cell label hPAP and synaptic markers. Vibratome slices were stained for hPAP, and processed for electron microscopy. Visual responses were recorded by electrophysiology from the superior colliculus (SC) in 12 rats at the age of 5.3-11.8 months. All recorded transplanted rats had restored or preserved visual responses in the SC corresponding to the transplant location in the retina, with thresholds between -2.8 and -3.4 log cd/m(2). No such responses were found in age-matched S334ter-3 rats without transplants, or in those with sham surgery. Donor cells and processes were identified in the host by light and electron microscopy. Transplant processes penetrated the inner host retina in spite of occasional glial barriers between transplant and host. Labeled neuronal processes were found in the host inner plexiform layer, and formed apparent synapses with unlabeled cells, presumably of host origin. In conclusion, synaptic connections between graft and host cells, together with visual responses from corresponding locations in the brain, support the hypothesis that functional connections develop following transplantation of retinal layers into rodent models of retinal degeneration.
Figures



Similar articles
-
Structure and function of embryonic rat retinal sheet transplants.Curr Eye Res. 2007 Sep;32(9):781-9. doi: 10.1080/02713680701530597. Curr Eye Res. 2007. PMID: 17882711
-
Retinal transplants restore visual responses: trans-synaptic tracing from visually responsive sites labels transplant neurons.Eur J Neurosci. 2008 Jul;28(1):208-20. doi: 10.1111/j.1460-9568.2008.06279.x. Eur J Neurosci. 2008. PMID: 18662343
-
BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function.Exp Eye Res. 2008 Jan;86(1):92-104. doi: 10.1016/j.exer.2007.09.012. Epub 2007 Oct 2. Exp Eye Res. 2008. PMID: 17983616 Free PMC article.
-
Cell replacement and visual restoration by retinal sheet transplants.Prog Retin Eye Res. 2012 Nov;31(6):661-87. doi: 10.1016/j.preteyeres.2012.06.003. Epub 2012 Jul 5. Prog Retin Eye Res. 2012. PMID: 22771454 Free PMC article. Review.
-
Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function.Semin Ophthalmol. 2005 Jan-Mar;20(1):31-42. doi: 10.1080/08820530590921873. Semin Ophthalmol. 2005. PMID: 15804842 Review.
Cited by
-
Self-organizing optic-cup morphogenesis in three-dimensional culture.Nature. 2011 Apr 7;472(7341):51-6. doi: 10.1038/nature09941. Nature. 2011. PMID: 21475194
-
Detailed Visual Cortical Responses Generated by Retinal Sheet Transplants in Rats with Severe Retinal Degeneration.J Neurosci. 2018 Dec 12;38(50):10709-10724. doi: 10.1523/JNEUROSCI.1279-18.2018. Epub 2018 Nov 5. J Neurosci. 2018. PMID: 30396913 Free PMC article.
-
Material Exchange in Photoreceptor Transplantation: Updating Our Understanding of Donor/Host Communication and the Future of Cell Engraftment Science.Front Neural Circuits. 2018 Mar 6;12:17. doi: 10.3389/fncir.2018.00017. eCollection 2018. Front Neural Circuits. 2018. PMID: 29559897 Free PMC article. Review.
-
Stem cells as a therapeutic tool for the blind: biology and future prospects.Proc Biol Sci. 2011 Oct 22;278(1721):3009-16. doi: 10.1098/rspb.2011.1028. Epub 2011 Aug 3. Proc Biol Sci. 2011. PMID: 21813553 Free PMC article. Review.
-
Stem cells for retinal replacement therapy.Neurotherapeutics. 2011 Oct;8(4):736-43. doi: 10.1007/s13311-011-0077-6. Neurotherapeutics. 2011. PMID: 21948217 Free PMC article. Review.
References
-
- Arai S, Thomas BB, Seiler MJ, Aramant RB, Qiu G, Mui C, de Juan E, Sadda SR. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp Eye Res. 2004;79:331–341. - PubMed
-
- Aramant RB, Seiler MJ. Retinal Transplantation - Advantages of Intact Fetal Sheets. Prog Retin Eye Res. 2002;21:57–73. - PubMed
-
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. - PubMed
-
- Brandstatter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. - PubMed
-
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

