Activated Ras alters lens and corneal development through induction of distinct downstream targets
- PMID: 20105280
- PMCID: PMC2828409
- DOI: 10.1186/1471-213X-10-13
Activated Ras alters lens and corneal development through induction of distinct downstream targets
Abstract
Background: Mammalian Ras genes regulate diverse cellular processes including proliferation and differentiation and are frequently mutated in human cancers. Tumor development in response to Ras activation varies between different tissues and the molecular basis for these variations are poorly understood. The murine lens and cornea have a common embryonic origin and arise from adjacent regions of the surface ectoderm. Activation of the fibroblast growth factor (FGF) signaling pathway induces the corneal epithelial cells to proliferate and the lens epithelial cells to exit the cell cycle. The molecular mechanisms that regulate the differential responses of these two related tissues have not been defined. We have generated transgenic mice that express a constitutively active version of human H-Ras in their lenses and corneas.
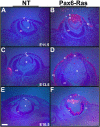
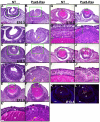
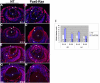
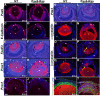
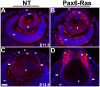
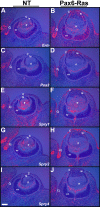
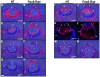
Results: Ras transgenic lenses and corneal epithelial cells showed increased proliferation with concomitant increases in cyclin D1 and D2 expression. This initial increase in proliferation is sustained in the cornea but not in the lens epithelial cells. Coincidentally, cdk inhibitors p27Kip1 and p57Kip2 were upregulated in the Ras transgenic lenses but not in the corneas. Phospho-Erk1 and Erk2 levels were elevated in the lens but not in the cornea and Spry 1 and Spry 2, negative regulators of Ras-Raf-Erk signaling, were upregulated more in the corneal than in the lens epithelial cells. Both lens and corneal differentiation programs were sensitive to Ras activation. Ras transgenic embryos showed a distinctive alteration in the architecture of the lens pit. Ras activation, though sufficient for upregulation of Prox1, a transcription factor critical for cell cycle exit and initiation of fiber differentiation, is not sufficient for induction of terminal fiber differentiation. Expression of Keratin 12, a marker of corneal epithelial differentiation, was reduced in the Ras transgenic corneas.
Conclusions: Collectively, these results suggest that Ras activation a) induces distinct sets of downstream targets in the lens and cornea resulting in distinct cellular responses and b) is sufficient for initiation but not completion of lens fiber differentiation.
Figures
Similar articles
-
Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation.Invest Ophthalmol Vis Sci. 2011 Aug 29;52(9):6887-97. doi: 10.1167/iovs.11-7531. Invest Ophthalmol Vis Sci. 2011. PMID: 21743007 Free PMC article.
-
Activated Ras induces lens epithelial cell hyperplasia but not premature differentiation.Int J Dev Biol. 2004;48(8-9):879-88. doi: 10.1387/ijdb.041889lr. Int J Dev Biol. 2004. PMID: 15558479
-
Ras signaling is essential for lens cell proliferation and lens growth during development.Dev Biol. 2006 Oct 15;298(2):403-14. doi: 10.1016/j.ydbio.2006.06.045. Epub 2006 Jul 4. Dev Biol. 2006. PMID: 16889766
-
Coordinating cell proliferation and migration in the lens and cornea.Semin Cell Dev Biol. 2008 Apr;19(2):113-24. doi: 10.1016/j.semcdb.2007.10.001. Epub 2007 Oct 18. Semin Cell Dev Biol. 2008. PMID: 18035561 Review.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
Cited by
-
Glycosaminoglycan-dependent restriction of FGF diffusion is necessary for lacrimal gland development.Development. 2012 Aug;139(15):2730-9. doi: 10.1242/dev.079236. Epub 2012 Jun 28. Development. 2012. PMID: 22745308 Free PMC article.
-
Jack of all trades, master of each: the diversity of fibroblast growth factor signalling in eye development.Open Biol. 2022 Jan;12(1):210265. doi: 10.1098/rsob.210265. Epub 2022 Jan 12. Open Biol. 2022. PMID: 35016551 Free PMC article. Review.
-
Multiomic analysis implicates FOXO4 in genetic regulation of chick lens fiber cell differentiation.Dev Biol. 2023 Dec;504:25-37. doi: 10.1016/j.ydbio.2023.09.005. Epub 2023 Sep 16. Dev Biol. 2023. PMID: 37722500 Free PMC article.
-
Frs2α enhances fibroblast growth factor-mediated survival and differentiation in lens development.Development. 2012 Dec;139(24):4601-12. doi: 10.1242/dev.081737. Epub 2012 Nov 7. Development. 2012. PMID: 23136392 Free PMC article.
-
Expression of truncated PITX3 in the developing lens leads to microphthalmia and aphakia in mice.PLoS One. 2014 Oct 27;9(10):e111432. doi: 10.1371/journal.pone.0111432. eCollection 2014. PLoS One. 2014. PMID: 25347445 Free PMC article.
References
-
- Donovan S, Shannon KM, Bollag G. GTPase activating proteins: critical regulators of intracellular signaling. Biochim Biophys Acta. 2002;1602:23–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

