Comparative distribution of human and avian type sialic acid influenza receptors in the pig
- PMID: 20105300
- PMCID: PMC2832630
- DOI: 10.1186/1746-6148-6-4
Comparative distribution of human and avian type sialic acid influenza receptors in the pig
Abstract
Background: A major determinant of influenza infection is the presence of virus receptors on susceptible host cells to which the viral haemagglutinin is able to bind. Avian viruses preferentially bind to sialic acid alpha2,3-galactose (SAalpha2,3-Gal) linked receptors, whereas human strains bind to sialic acid alpha2,6-galactose (SAalpha2,6-Gal) linked receptors. To date, there has been no detailed account published on the distribution of SA receptors in the pig, a model host that is susceptible to avian and human influenza subtypes, thus with potential for virus reassortment. We examined the relative expression and spatial distribution of SAalpha2,3-GalG(1-3)GalNAc and SAalpha2,6-Gal receptors in the major organs from normal post-weaned pigs by binding with lectins Maackia amurensis agglutinins (MAA II) and Sambucus nigra agglutinin (SNA) respectively.
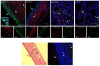
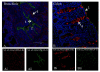
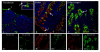
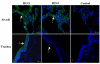
Results: Both SAalpha2,3-Gal and SAalpha2,6-Gal receptors were extensively detected in the major porcine organs examined (trachea, lung, liver, kidney, spleen, heart, skeletal muscle, cerebrum, small intestine and colon). Furthermore, distribution of both SA receptors in the pig respiratory tract closely resembled the published data of the human tract. Similar expression patterns of SA receptors between pig and human in other major organs were found, with exception of the intestinal tract. Unlike the limited reports on the scarcity of influenza receptors in human intestines, we found increasing presence of SAalpha2,3-Gal and SAalpha2,6-Gal receptors from duodenum to colon in the pig.
Conclusions: The extensive presence of SAalpha2,3-Gal and SAalpha2,6-Gal receptors in the major organs examined suggests that each major organ may be permissive to influenza virus entry or infection. The high similarity of SA expression patterns between pig and human, in particular in the respiratory tract, suggests that pigs are not more likely to be potential hosts for virus reassortment than humans. Our finding of relative abundance of SA receptors in the pig intestines highlights a need for clarification on the presence of SA receptors in the human intestinal tract.
Figures


Similar articles
-
Differences in influenza virus receptors in chickens and ducks: Implications for interspecies transmission.J Mol Genet Med. 2009 Jan 16;3(1):143-51. doi: 10.4172/1747-0862.1000026. J Mol Genet Med. 2009. PMID: 19565022 Free PMC article.
-
Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses.Respir Res. 2007 Oct 25;8(1):73. doi: 10.1186/1465-9921-8-73. Respir Res. 2007. PMID: 17961210 Free PMC article.
-
The distribution of sialic acid receptors of avian influenza virus in the reproductive tract of laying hens.Mol Cell Probes. 2015 Apr;29(2):129-34. doi: 10.1016/j.mcp.2015.01.002. Epub 2015 Feb 26. Mol Cell Probes. 2015. PMID: 25725345
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
[Receptor sialylsugar chains as determinants of host range of influenza viruses].Nihon Rinsho. 2000 Nov;58(11):2206-10. Nihon Rinsho. 2000. PMID: 11225305 Review. Japanese.
Cited by
-
Emergence of fatal avian influenza in New England harbor seals.mBio. 2012 Jul 31;3(4):e00166-12. doi: 10.1128/mBio.00166-12. Print 2012. mBio. 2012. PMID: 22851656 Free PMC article.
-
Transmission of influenza A viruses.Virology. 2015 May;479-480:234-46. doi: 10.1016/j.virol.2015.03.009. Epub 2015 Mar 24. Virology. 2015. PMID: 25812763 Free PMC article. Review.
-
Evidence of influenza A infection and risk of transmission between pigs and farmworkers.Zoonoses Public Health. 2022 Aug;69(5):560-571. doi: 10.1111/zph.12948. Epub 2022 Apr 20. Zoonoses Public Health. 2022. PMID: 35445551 Free PMC article.
-
The Betacoronavirus PHEV Replicates and Disrupts the Respiratory Epithelia and Upregulates Key Pattern Recognition Receptor Genes and Downstream Mediators, Including IL-8 and IFN-λ.mSphere. 2021 Dec 22;6(6):e0082021. doi: 10.1128/mSphere.00820-21. Epub 2021 Dec 22. mSphere. 2021. PMID: 34935443 Free PMC article.
-
Influenza A virus transmission via respiratory aerosols or droplets as it relates to pandemic potential.FEMS Microbiol Rev. 2016 Jan;40(1):68-85. doi: 10.1093/femsre/fuv039. Epub 2015 Sep 17. FEMS Microbiol Rev. 2016. PMID: 26385895 Free PMC article. Review.
References
-
- Olsen CW, Brown IH, Easterday BC, Van Reeth K. In: Diseases of swine. 9. Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editor. Oxford: Blackwell Publishing; 2006. Swine influenza; pp. 469–482.
-
- Brookes SM, Irvine RM, Nunez A, Clifford D, Essen S, Brown IH. Influenza A (H1N1) infection in pigs. Vet Rec. 2009;164:760–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

