Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation
- PMID: 20105334
- PMCID: PMC2842246
- DOI: 10.1186/1479-5876-8-7
Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation
Abstract
Background: The conventional treatment protocol in high-intensity focused ultrasound (HIFU) therapy utilizes a dense-scan strategy to produce closely packed thermal lesions aiming at eradicating as much tumor mass as possible. However, this strategy is not most effective in terms of inducing a systemic anti-tumor immunity so that it cannot provide efficient micro-metastatic control and long-term tumor resistance. We have previously provided evidence that HIFU may enhance systemic anti-tumor immunity by in situ activation of dendritic cells (DCs) inside HIFU-treated tumor tissue. The present study was conducted to test the feasibility of a sparse-scan strategy to boost HIFU-induced anti-tumor immune response by more effectively promoting DC maturation.
Methods: An experimental HIFU system was set up to perform tumor ablation experiments in subcutaneous implanted MC-38 and B16 tumor with dense- or sparse-scan strategy to produce closely-packed or separated thermal lesions. DCs infiltration into HIFU-treated tumor tissues was detected by immunohistochemistry and flow cytometry. DCs maturation was evaluated by IL-12/IL-10 production and CD80/CD86 expression after co-culture with tumor cells treated with different HIFU. HIFU-induced anti-tumor immune response was evaluated by detecting growth-retarding effects on distant re-challenged tumor and tumor-specific IFN-gamma-secreting cells in HIFU-treated mice.
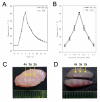
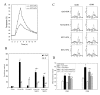
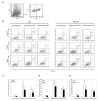
Results: HIFU exposure raised temperature up to 80 degrees centigrade at beam focus within 4 s in experimental tumors and led to formation of a well-defined thermal lesion. The infiltrated DCs were recruited to the periphery of lesion, where the peak temperature was only 55 degrees centigrade during HIFU exposure. Tumor cells heated to 55 degrees centigrade in 4-s HIFU exposure were more effective to stimulate co-cultured DCs to mature. Sparse-scan HIFU, which can reserve 55 degrees-heated tumor cells surrounding the separated lesions, elicited an enhanced anti-tumor immune response than dense-scan HIFU, while their suppressive effects on the treated primary tumor were maintained at the same level. Flow cytometry analysis showed that sparse-scan HIFU was more effective than dense-scan HIFU in enhancing DC infiltration into tumor tissues and promoting their maturation in situ.
Conclusion: Optimizing scan strategy is a feasible way to boost HIFU-induced anti-tumor immunity by more effectively promoting DC maturation.
Figures



References
-
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, Baum J, Singer S, Jolesz FA. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219:176–185. - PubMed
-
- Chen W, Wang Z, Wu F, Zhu H, Zou J, Bai J, Li K, Xie F. [High intensity focused ultrasound in the treatment of primary malignant bone tumor] Zhonghua Zhong Liu Za Zhi. 2002;24:612–615. - PubMed
-
- Zhang L, Chen WZ, Liu YJ, Hu X, Zhou K, Chen L, Peng S, Zhu H, Zou HL, Bai J, Wang ZB. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

