Defining hepatoblastoma responsiveness to induction therapy as measured by tumor volume and serum alpha-fetoprotein kinetics
- PMID: 20105591
- PMCID: PMC2852870
- DOI: 10.1016/j.jpedsurg.2009.10.023
Defining hepatoblastoma responsiveness to induction therapy as measured by tumor volume and serum alpha-fetoprotein kinetics
Abstract
Purpose: Hepatoblastoma is commonly unresectable at presentation, necessitating induction chemotherapy before definitive resection. To refine the paradigm for timing of resection, we questioned whether a plateau in hepatoblastoma responsiveness to neoadjuvant therapy could be detected by calculating tumor volume (TV) and serum alpha-fetoprotein (sAFP) kinetics.
Methods: To calculate TV and sAFP as measures of treatment responsiveness over time, infants having initially unresectable epithelial-type hepatoblastomas were identified at a single institution (1996-2008). Effects of therapy type, therapy duration, and lobe of liver involvement on TV, sAFP, margin status, and toxicity were analyzed.
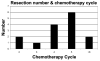
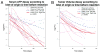
Results: Of 24 infants treated for epithelial-type hepatoblastoma during this interval, 5 were resected primarily, and 15 had complete digital films for kinetics analysis. Both TV and sAFP decreased dramatically over time (P < .0001). No statistically significant difference in mean TV or sAFP was detected after chemotherapy cycle 2. Left lobe tumors had greater presenting levels of and significantly slower decay in sAFP compared with right lobe tumors (P = .005), although no statistically significant differences in TV existed between liver lobes. Resection margins did not change with therapy duration.
Conclusions: Measuring TV and sAFP kinetics accurately reflects hepatoblastoma responsiveness to induction therapy. Treatment toxicities may be reduced by earlier resection and tailoring of chemotherapeutic regimens.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Rapid decrease of serum alpha-fetoprotein and tumor volume predicts outcome in children with hepatoblastoma treated with neoadjuvant chemotherapy.Int J Clin Oncol. 2018 Oct;23(5):900-907. doi: 10.1007/s10147-018-1285-4. Epub 2018 May 9. Int J Clin Oncol. 2018. PMID: 29744604
-
Survival after liver transplantation for hepatoblastoma: a 2-center experience.J Pediatr Surg. 2008 Nov;43(11):1973-81. doi: 10.1016/j.jpedsurg.2008.05.031. J Pediatr Surg. 2008. PMID: 18970927
-
[Differentiated treatment protocols for high- and standard-risk hepatoblastoma--an interim report of the German Liver Tumor Study HB99].Klin Padiatr. 2003 May-Jun;215(3):159-65. doi: 10.1055/s-2003-39375. Klin Padiatr. 2003. PMID: 12778356 Clinical Trial. German.
-
Efficacy of neoadjuvant therapy and surgical rescue for locally advanced hepatoblastomas: 10 year single-center experience and literature review.World J Gastroenterol. 2014 Aug 7;20(29):10137-43. doi: 10.3748/wjg.v20.i29.10137. World J Gastroenterol. 2014. PMID: 25110441 Free PMC article. Review.
-
Hepatoblastoma--evolution of management and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases.J Pediatr Surg. 2004 Sep;39(9):1321-7. doi: 10.1016/j.jpedsurg.2004.05.020. J Pediatr Surg. 2004. PMID: 15359384 Review.
Cited by
-
Surgical Management of Hepatoblastoma and Recent Advances.Cancers (Basel). 2019 Dec 4;11(12):1944. doi: 10.3390/cancers11121944. Cancers (Basel). 2019. PMID: 31817219 Free PMC article. Review.
-
Imaging analysis of hepatoblastoma resectability across neoadjuvant chemotherapy.J Pediatr Surg. 2013 Jun;48(6):1239-48. doi: 10.1016/j.jpedsurg.2013.03.019. J Pediatr Surg. 2013. PMID: 23845613 Free PMC article.
-
Impact of preoperative chemotherapy cycles on tumor resectability and surgical timing in hepatoblastoma: a retrospective analysis.World J Pediatr Surg. 2025 Jun 19;8(3):e000997. doi: 10.1136/wjps-2024-000997. eCollection 2025. World J Pediatr Surg. 2025. PMID: 40546418 Free PMC article.
-
Association of image-defined risk factors with clinical features, tumor biology, and outcomes in neuroblastoma: a single-center retrospective study.Eur J Pediatr. 2023 May;182(5):2189-2196. doi: 10.1007/s00431-023-04899-0. Epub 2023 Mar 1. Eur J Pediatr. 2023. PMID: 36856889
-
Quantitative ctDNA Detection in Hepatoblastoma: Implications for Precision Medicine.Cancers (Basel). 2023 Dec 19;16(1):12. doi: 10.3390/cancers16010012. Cancers (Basel). 2023. PMID: 38201440 Free PMC article.
References
-
- Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008 Jul;13(7):812–20. - PubMed
-
- Meyers RL. Tumors of the liver in children. Surg Oncol. 2007 Nov;16(3):195–203. - PubMed
-
- Herzog CE, Andrassy RJ, Eftekhari F. Childhood cancers: hepatoblastoma. Oncologist. 2000;5(6):445–53. - PubMed
-
- Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000 Jul;18(14):2665–75. - PubMed
-
- Evans AE, Land VJ, Newton WA, Randolph JG, Sather HN, Tefft M. Combination chemotherapy (vincristine, adriamycin, cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer. 1982 Sep 1;50(5):821–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

