Principles governing oligomer formation in amyloidogenic peptides
- PMID: 20106655
- PMCID: PMC2854190
- DOI: 10.1016/j.sbi.2009.12.017
Principles governing oligomer formation in amyloidogenic peptides
Abstract
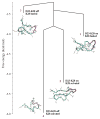
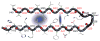
Identifying the principles that describe the formation of protein oligomers and fibrils with distinct morphologies is a daunting problem. Here we summarize general principles of oligomer formation gleaned from molecular dynamics simulations of Abeta-peptides. The spectra of high free energy structures sampled by the monomer provide insights into the plausible fibril structures, providing a rationale for the 'strain phenomenon.' Heterogeneous growth dynamics of small oligomers of Abeta(16-22), whose lowest free energy structures are like nematic droplets, can be broadly described using a two-stage dock-lock mechanism. In the growth process, water is found to play various roles depending on the oligomer size, and peptide length, and sequence. Water may be an explicit element of fibril structure linked to various fibril morphologies.
Figures
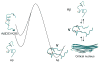
Similar articles
-
Toward a molecular theory of early and late events in monomer to amyloid fibril formation.Annu Rev Phys Chem. 2011;62:437-63. doi: 10.1146/annurev-physchem-032210-103526. Annu Rev Phys Chem. 2011. PMID: 21219143 Free PMC article. Review.
-
Role of water in protein aggregation and amyloid polymorphism.Acc Chem Res. 2012 Jan 17;45(1):83-92. doi: 10.1021/ar2000869. Epub 2011 Jul 15. Acc Chem Res. 2012. PMID: 21761818 Free PMC article.
-
Influence of preformed Asp23-Lys28 salt bridge on the conformational fluctuations of monomers and dimers of Abeta peptides with implications for rates of fibril formation.J Phys Chem B. 2009 Jan 29;113(4):1162-72. doi: 10.1021/jp808914c. J Phys Chem B. 2009. PMID: 19125574 Free PMC article.
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
Elucidating Important Sites and the Mechanism for Amyloid Fibril Formation by Coarse-Grained Molecular Dynamics.ACS Chem Neurosci. 2017 Jan 18;8(1):201-209. doi: 10.1021/acschemneuro.6b00331. Epub 2016 Nov 18. ACS Chem Neurosci. 2017. PMID: 28095675 Free PMC article.
Cited by
-
Aggregation of γ-crystallins associated with human cataracts via domain swapping at the C-terminal β-strands.Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10514-9. doi: 10.1073/pnas.1019152108. Epub 2011 Jun 13. Proc Natl Acad Sci U S A. 2011. PMID: 21670251 Free PMC article.
-
Exploring the role of hydration and confinement in the aggregation of amyloidogenic peptides Aβ(16-22) and Sup35(7-13) in AOT reverse micelles.J Chem Phys. 2014 Dec 14;141(22):22D530. doi: 10.1063/1.4902550. J Chem Phys. 2014. PMID: 25494801 Free PMC article.
-
Molecular mechanism of misfolding and aggregation of Aβ(13-23).J Phys Chem B. 2013 May 23;117(20):6175-86. doi: 10.1021/jp402938p. Epub 2013 May 15. J Phys Chem B. 2013. PMID: 23642026 Free PMC article.
-
Toward a molecular theory of early and late events in monomer to amyloid fibril formation.Annu Rev Phys Chem. 2011;62:437-63. doi: 10.1146/annurev-physchem-032210-103526. Annu Rev Phys Chem. 2011. PMID: 21219143 Free PMC article. Review.
-
Effects of environmental factors on MSP21-25 aggregation indicate the roles of hydrophobic and electrostatic interactions in the aggregation process.Eur Biophys J. 2014 Jan;43(1):1-9. doi: 10.1007/s00249-013-0934-9. Epub 2013 Oct 23. Eur Biophys J. 2014. PMID: 24150738
References
-
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. Aβ dimers were shown to be the cause of long term potentiation and long term depression in rats. Learning behavior in rats was greatly disrupted by injection of soluble Aβ-dimers. Taken together these studies show that soluble oligomers by themselves can lead to many of the symptoms associated with AD. - PMC - PubMed
-
- Wolfe M, Guénette SY. APP at a glance. J Cell Sci. 2007;120:3157–3161. - PubMed
-
- Roher AE, Ball MJ, Bhave SV, Wakade AR. β-Amyloid from Alzheimer disease brains inhibits sprouting and survival of sympathetic neurons. Biochem Biophys Res Commun. 1991;174:572–579. - PubMed
-
- Glenner GG, Wong CW. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Lee JP, Stimson ER, Ghilardi JR, Mantyh PW, Lu YA, Felix AM, Llanos W, Behbin A, Cummings M, Criekinge MV, Timms W, Maggio JE. 1H NMR of Aβ amyloid peptide congeners in water solution. Conformational changes correlate with plaque competence. Biochemistry. 1995;34:5191–5200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

