Avian magnetite-based magnetoreception: a physiologist's perspective
- PMID: 20106875
- PMCID: PMC2844004
- DOI: 10.1098/rsif.2009.0423.focus
Avian magnetite-based magnetoreception: a physiologist's perspective
Abstract
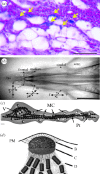
It is now well established that animals use the Earth's magnetic field to perform long-distance migration and other navigational tasks. However, the transduction mechanisms that allow the conversion of magnetic field variations into an electric signal by specialized sensory cells remain largely unknown. Among the species that have been shown to sense Earth-strength magnetic fields, birds have been a model of choice since behavioural tests show that their direction-finding abilities are strongly influenced by magnetic fields. Magnetite, a ferromagnetic mineral, has been found in a wide range of organisms, from bacteria to vertebrates. In birds, both superparamagnetic (SPM) and single-domain magnetite have been found to be associated with the trigeminal nerve. Electrophysiological recordings from cells in the trigeminal ganglion have shown an increase in action potential firing in response to magnetic field changes. More recently, histological evidence has demonstrated the presence of SPM magnetite in the subcutis of the pigeon's upper beak. The aims of the present review are to review the evidence for a magnetite-based mechanism in birds and to introduce physiological concepts in order to refine the proposed models.
Figures

Similar articles
-
The magnetite-based receptors in the beak of birds and their role in avian navigation.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Feb;199(2):89-98. doi: 10.1007/s00359-012-0769-3. Epub 2012 Oct 31. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23111859 Free PMC article. Review.
-
Directional orientation of birds by the magnetic field under different light conditions.J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S163-77. doi: 10.1098/rsif.2009.0367.focus. Epub 2009 Oct 28. J R Soc Interface. 2010. PMID: 19864263 Free PMC article. Review.
-
Biophysics of magnetic orientation: strengthening the interface between theory and experimental design.J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S179-91. doi: 10.1098/rsif.2009.0491.focus. Epub 2010 Jan 13. J R Soc Interface. 2010. PMID: 20071390 Free PMC article.
-
Cryptochromes--a potential magnetoreceptor: what do we know and what do we want to know?J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S147-62. doi: 10.1098/rsif.2009.0411.focus. Epub 2009 Nov 11. J R Soc Interface. 2010. PMID: 19906675 Free PMC article.
-
Magnetoreception in birds.J R Soc Interface. 2019 Sep 27;16(158):20190295. doi: 10.1098/rsif.2019.0295. Epub 2019 Sep 4. J R Soc Interface. 2019. PMID: 31480921 Free PMC article. Review.
Cited by
-
Cryptochromes in Mammals and Birds: Clock or Magnetic Compass?Physiology (Bethesda). 2021 May 1;36(3):183-194. doi: 10.1152/physiol.00040.2020. Physiology (Bethesda). 2021. PMID: 33904789 Free PMC article.
-
Magnetic particle-mediated magnetoreception.J R Soc Interface. 2015 Sep 6;12(110):0499. doi: 10.1098/rsif.2015.0499. J R Soc Interface. 2015. PMID: 26333810 Free PMC article. Review.
-
Biophysical mechanism of animal magnetoreception, orientation and navigation.Sci Rep. 2024 Dec 3;14(1):30053. doi: 10.1038/s41598-024-77883-9. Sci Rep. 2024. PMID: 39627252 Free PMC article. Review.
-
Hypothetical superparamagnetic magnetometer in a pigeon's upper beak probably does not work.Eur Phys J E Soft Matter. 2013 Apr;36(4):9853. doi: 10.1140/epje/i2013-13040-1. Epub 2013 Apr 23. Eur Phys J E Soft Matter. 2013. PMID: 23605568
-
Brain-to-brain communication: the possible role of brain electromagnetic fields (As a Potential Hypothesis).Heliyon. 2021 Mar 1;7(3):e06363. doi: 10.1016/j.heliyon.2021.e06363. eCollection 2021 Mar. Heliyon. 2021. PMID: 33732922 Free PMC article. Review.
References
-
- Beason R. C., Brennan W. J. 1986. Natural and induced magnetization in the bobolink, Dolichonyx oryzivorus (Aves: Icteridae). J. Exp. Biol. 125, 49–56.
-
- Beason R. C., Nichols J. E. 1984. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309, 151–153. (10.1038/309151a0) - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

