Escherichia coli rpoB mutants have increased evolvability in proportion to their fitness defects
- PMID: 20106907
- PMCID: PMC2872623
- DOI: 10.1093/molbev/msq024
Escherichia coli rpoB mutants have increased evolvability in proportion to their fitness defects
Abstract
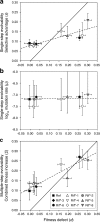
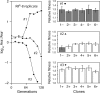
Evolvability is the capacity of an organism or population for generating descendants with increased fitness. Simulations and comparative studies have shown that evolvability can vary among individuals and identified characteristics of genetic architectures that can promote evolvability. However, little is known about how the evolvability of biological organisms typically varies along a lineage at each mutational step in its history. Evolvability might increase upon sustaining a deleterious mutation because there are many compensatory paths in the fitness landscape to reascend the same fitness peak or because shifts to new peaks become possible. We use genetic marker divergence trajectories to parameterize and compare the evolvability--defined as the fitness increase realized by an evolving population initiated from a test genotype--of a series of Escherichia coli mutants on multiple timescales. Each mutant differs from a common progenitor strain by a mutation in the rpoB gene, which encodes the beta subunit of RNA polymerase. Strains with larger fitness defects are proportionally more evolvable in terms of both the beneficial mutations accessible in their immediate mutational neighborhoods and integrated over evolutionary paths that traverse multiple beneficial mutations. Our results establish quantitative expectations for how a mutation with a given deleterious fitness effect should influence evolvability, and they will thus inform future studies of how deleterious, neutral, and beneficial mutations targeting other cellular processes impact the evolutionary potential of microorganisms.
Figures

References
-
- Ahrens JH, Dieter U. Computer generation of Poisson deviates from modified normal distributions. ACM Trans Math Software. 1982;8:163–179.
-
- Barrick JE, Yu DS, Yoon SH, Jeong H, Oh TK, Schneider D, Lenski RE, Kim JF. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature. 2009;461:1243–1247. - PubMed
-
- Bebbington AC. A simple method of drawing a sample without replacement. Appl Statist. 1975;24:136.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

