Efficient budding of the tacaribe virus matrix protein z requires the nucleoprotein
- PMID: 20106925
- PMCID: PMC2838133
- DOI: 10.1128/JVI.02429-09
Efficient budding of the tacaribe virus matrix protein z requires the nucleoprotein
Abstract
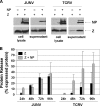
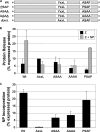
The Z protein has been shown for several arenaviruses to serve as the viral matrix protein. As such, Z provides the principal force for the budding of virus particles and is capable of forming virus-like particles (VLPs) when expressed alone. For most arenaviruses, this activity has been shown to be linked to the presence of proline-rich late-domain motifs in the C terminus; however, for the New World arenavirus Tacaribe virus (TCRV), no such motif exists within Z. It was recently demonstrated that while TCRV Z is still capable of functioning as a matrix protein to induce the formation of VLPs, neither its ASAP motif, which replaces a canonical PT/SAP motif in related viruses, nor its YxxL motif is involved in budding, leading to the suggestion that TCRV uses a novel budding mechanism. Here we show that in comparison to its closest relative, Junin virus (JUNV), TCRV Z buds only weakly when expressed in isolation. While this budding activity is independent of the ASAP or YxxL motif, it is significantly enhanced by coexpression with the nucleoprotein (NP), an effect not seen with JUNV Z. Interestingly, both the ASAP and YxxL motifs of Z appear to be critical for the recruitment of NP into VLPs, as well as for the enhancement of TCRV Z-mediated budding. While it is known that TCRV budding remains dependent on the endosomal sorting complex required for transport, our findings provide further evidence that TCRV uses a budding mechanism distinct from that of other known arenaviruses and suggest an essential role for NP in this process.
Figures



Similar articles
-
Arenavirus budding: a common pathway with mechanistic differences.Viruses. 2013 Jan 31;5(2):528-49. doi: 10.3390/v5020528. Viruses. 2013. PMID: 23435234 Free PMC article. Review.
-
The z protein of the new world arenavirus tacaribe virus has bona fide budding activity that does not depend on known late domain motifs.J Virol. 2009 Dec;83(23):12651-5. doi: 10.1128/JVI.01012-09. Epub 2009 Sep 16. J Virol. 2009. PMID: 19759156 Free PMC article.
-
Identification of critical amino acids within the nucleoprotein of Tacaribe virus important for anti-interferon activity.J Biol Chem. 2013 Mar 22;288(12):8702-8711. doi: 10.1074/jbc.M112.444760. Epub 2013 Feb 4. J Biol Chem. 2013. PMID: 23382389 Free PMC article.
-
The RING domain and the L79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of glycoproteins into infectious chimeric arenavirus-like particles.J Virol. 2009 Jul;83(14):7029-39. doi: 10.1128/JVI.00329-09. Epub 2009 May 6. J Virol. 2009. PMID: 19420075 Free PMC article.
-
[Envelope virus assembly and budding].Uirusu. 2010 Jun;60(1):105-13. doi: 10.2222/jsv.60.105. Uirusu. 2010. PMID: 20848870 Review. Japanese.
Cited by
-
Arenavirus budding: a common pathway with mechanistic differences.Viruses. 2013 Jan 31;5(2):528-49. doi: 10.3390/v5020528. Viruses. 2013. PMID: 23435234 Free PMC article. Review.
-
Lifecycle modelling systems support inosine monophosphate dehydrogenase (IMPDH) as a pro-viral factor and antiviral target for New World arenaviruses.Antiviral Res. 2018 Sep;157:140-150. doi: 10.1016/j.antiviral.2018.07.009. Epub 2018 Jul 19. Antiviral Res. 2018. PMID: 30031760 Free PMC article.
-
Molecular mechanism of arenavirus assembly and budding.Viruses. 2012 Oct 10;4(10):2049-79. doi: 10.3390/v4102049. Viruses. 2012. PMID: 23202453 Free PMC article. Review.
-
Differential contributions of tacaribe arenavirus nucleoprotein N-terminal and C-terminal residues to nucleocapsid functional activity.J Virol. 2014 Jun;88(11):6492-505. doi: 10.1128/JVI.00321-14. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696466 Free PMC article.
-
Autophagy Promotes Infectious Particle Production of Mopeia and Lassa Viruses.Viruses. 2019 Mar 23;11(3):293. doi: 10.3390/v11030293. Viruses. 2019. PMID: 30909570 Free PMC article.
References
-
- Bieniasz, P. D. 2006. Late budding domains and host proteins in enveloped virus release. Virology 344:55-63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

