Pharmacological manipulation of the akt signaling pathway regulates myxoma virus replication and tropism in human cancer cells
- PMID: 20106927
- PMCID: PMC2838123
- DOI: 10.1128/JVI.02020-09
Pharmacological manipulation of the akt signaling pathway regulates myxoma virus replication and tropism in human cancer cells
Abstract
Viruses have evolved an assortment of mechanisms for regulating the Akt signaling pathway to establish a cellular environment more favorable for viral replication. Myxoma virus (MYXV) is a rabbit-specific poxvirus that encodes many immunomodulatory factors, including an ankyrin repeat-containing host range protein termed M-T5 that functions to regulate tropism of MYXV for rabbit lymphocytes and certain human cancer cells. MYXV permissiveness in these human cancer cells is dependent upon the direct interaction between M-T5 and Akt, which has been shown to induce the kinase activity of Akt. In this study, an array of compounds that selectively manipulate Akt signaling was screened and we show that only a subset of Akt inhibitors significantly decreased the ability of MYXV to replicate in previously permissive human cancer cells. Furthermore, reduced viral replication efficiency was correlated with lower levels of phosphorylated Akt. In contrast, the PP2A-specific phosphatase inhibitor okadaic acid promoted increased Akt kinase activation and rescued MYXV replication in human cancer cells that did not previously support viral replication. Finally, phosphorylation of Akt at residue Thr308 was shown to dictate the physical interaction between Akt and M-T5, which then leads to phosphorylation of Ser473 and permits productive MYXV replication in these human cancer cells. The results of this study further characterize the mechanism by which M-T5 exploits the Akt signaling cascade and affirms this interaction as a major tropism determinant that regulates the replication efficiency of MYXV in human cancer cells.
Figures
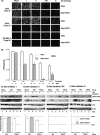
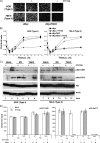


References
-
- Ahn, J. Y., R. Rong, T. G. Kroll, E. G. Van Meir, S. H. Snyder, and K. Ye. 2004. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J. Biol. Chem. 279:16441-16451. - PubMed
-
- Azuma, H., S. Takahara, S. Horie, S. Muto, Y. Otsuki, and Y. Katsuoka. 2003. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J. Urol. 169:2372-2377. - PubMed
-
- Azuma, H., S. Takahara, N. Ichimaru, J. D. Wang, Y. Itoh, Y. Otsuki, J. Morimoto, R. Fukui, M. Hoshiga, T. Ishihara, N. Nonomura, S. Suzuki, A. Okuyama, and Y. Katsuoka. 2002. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 62:1410-1419. - PubMed
-
- Barnett, S. F., D. Defeo-Jones, S. Fu, P. J. Hancock, K. M. Haskell, R. E. Jones, J. A. Kahana, A. M. Kral, K. Leander, L. L. Lee, J. Malinowski, E. M. McAvoy, D. D. Nahas, R. G. Robinson, and H. E. Huber. 2005. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 385:399-408. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

