Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene
- PMID: 20106950
- PMCID: PMC2855348
- DOI: 10.1093/toxsci/kfq022
Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene
Abstract
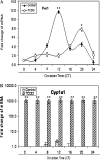
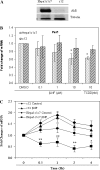
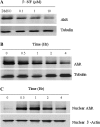
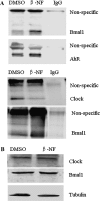
The aryl hydrocarbon receptor (AhR) is a period-aryl hydrocarbon receptor nuclear transporter-simple minded domain transcription factor that shares structural similarity with circadian clock genes and readily interacts with components of the molecular clock. Activation of AhR by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters behavioral circadian rhythms and represses the Period1 (Per1) gene in murine hematopoietic stem and progenitor cells. Per1 expression is driven by circadian locomotor activity cycles kaput-brain muscle ARNT-like (CLOCK-BMAL1)-dependent activation of Eboxes in the Per1 promoter. We hypothesized that the effects of AhR activation on the circadian clock are mediated by disruption of CLOCK-BMAL1 function and subsequent Per1 gene suppression. Effects of AhR activation on rhythmic Per1 transcripts were examined in livers of mice after treatment with the AhR agonist, TCDD; the molecular mechanisms of Per1 repression by AhR were determined in hepatoma cells using TCDD and beta-napthoflavone as AhR activators. This study reports, for the first time, that AhR activation by TCDD alters the Per1 rhythm in the mouse liver and that Per1 gene suppression depends upon the presence of AhR. Furthermore, AhR interaction with BMAL1 attenuates CLOCK-BMAL1 activity and decreases CLOCK binding at Ebox1 and Ebox3 in the Per1 promoter. Taken together, these data suggest that AhR activation represses Per1 through disrupting CLOCK-BMAL1 activity, producing dysregulation of rhythmic Per1 gene expression. These data define alteration of the Per1 rhythm as novel signaling events downstream of AhR activation. Downregulation of Per1 could contribute to metabolic disease, cancer, and other detrimental effects resulting from exposure to certain environmental pollutants.
Figures

Similar articles
-
Circadian clock disruption in the mouse ovary in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin.Toxicol Lett. 2011 Mar 5;201(2):116-22. doi: 10.1016/j.toxlet.2010.12.013. Epub 2010 Dec 21. Toxicol Lett. 2011. PMID: 21182907 Free PMC article.
-
Disruption of clock gene expression alters responses of the aryl hydrocarbon receptor signaling pathway in the mouse mammary gland.Mol Pharmacol. 2007 Nov;72(5):1349-58. doi: 10.1124/mol.107.039305. Epub 2007 Aug 22. Mol Pharmacol. 2007. PMID: 17715397
-
Disruption of period gene expression alters the inductive effects of dioxin on the AhR signaling pathway in the mouse liver.Toxicol Appl Pharmacol. 2009 Feb 1;234(3):370-7. doi: 10.1016/j.taap.2008.10.016. Epub 2008 Nov 7. Toxicol Appl Pharmacol. 2009. PMID: 19038280 Free PMC article.
-
Crosstalk between the AHR signaling pathway and circadian rhythm.Biochem Pharmacol. 2009 Feb 15;77(4):560-5. doi: 10.1016/j.bcp.2008.09.040. Epub 2008 Oct 15. Biochem Pharmacol. 2009. PMID: 18983986 Review.
-
The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship.Int J Mol Sci. 2023 Jan 12;24(2):1545. doi: 10.3390/ijms24021545. Int J Mol Sci. 2023. PMID: 36675058 Free PMC article. Review.
Cited by
-
Aryl hydrocarbon receptor affects circadian-regulated lipolysis through an E-Box-dependent mechanism.Mol Cell Endocrinol. 2023 Jan 1;559:111809. doi: 10.1016/j.mce.2022.111809. Epub 2022 Oct 23. Mol Cell Endocrinol. 2023. PMID: 36283500 Free PMC article.
-
Food-Borne Polycyclic Aromatic Hydrocarbons and Circadian Disruption.ACS Omega. 2024 Jul 9;9(29):31298-31312. doi: 10.1021/acsomega.4c04120. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072055 Free PMC article. Review.
-
Aryl Hydrocarbon Receptor Involvement in the Sodium-Dependent Glutamate/Aspartate Transporter Regulation in Cerebellar Bergmann Glia Cells.ACS Chem Neurosci. 2024 Mar 20;15(6):1276-1285. doi: 10.1021/acschemneuro.4c00046. Epub 2024 Mar 7. ACS Chem Neurosci. 2024. PMID: 38454572 Free PMC article.
-
Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction.Environ Health Insights. 2016 Aug 17;10:133-41. doi: 10.4137/EHI.S38343. eCollection 2016. Environ Health Insights. 2016. PMID: 27559298 Free PMC article. Review.
-
Interplay between Dioxin-mediated signaling and circadian clock: a possible determinant in metabolic homeostasis.Int J Mol Sci. 2014 Jul 1;15(7):11700-12. doi: 10.3390/ijms150711700. Int J Mol Sci. 2014. PMID: 24987953 Free PMC article. Review.
References
-
- Arisawa K, Takeda H, Mikasa H. Background exposure to PCDDs/PCDFs/PCBs and its potential health effects: a review of epidemiologic studies. J. Med. Invest. 2005;52:10–21. - PubMed
-
- Asai M, Yamaguchi S, Isejima H, Jonouchi M, Moriya T, Shibata S, Kobayashi M, Okamura H. Visualization of mPer1 transcription in vitro: NMDA induces a rapid phase shift of mPer1 gene in cultured SCN. Curr. Biol. 2001;11:1524–1527. - PubMed
-
- Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am. J. Physiol. 2003;285:R561–R569. - PubMed
-
- Bock KW. Aryl hydrocarbon or dioxin receptor: biologic and toxic responses. Rev. Physiol. Biochem. Pharmacol. 1994;125:1–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

