cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3
- PMID: 20106966
- PMCID: PMC2856983
- DOI: 10.1074/jbc.M109.034868
cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3
Abstract
The concentration of the second messenger cAMP is tightly controlled in cells by the activity of phosphodiesterases. We have previously described how the protein kinase A-anchoring protein mAKAP serves as a scaffold for the cAMP-dependent protein kinase PKA and the cAMP-specific phosphodiesterase PDE4D3 in cardiac myocytes. PKA and PDE4D3 constitute a negative feedback loop whereby PKA-catalyzed phosphorylation and activation of PDE4D3 attenuate local cAMP levels. We now show that protein phosphatase 2A (PP2A) associated with mAKAP complexes is responsible for reversing the activation of PDE4D3 by catalyzing the dephosphorylation of PDE4D3 serine residue 54. Mapping studies reveal that a C-terminal mAKAP domain (residues 2085-2319) binds PP2A. Binding to mAKAP is required for PP2A function, such that deletion of the C-terminal domain enhances both base-line and forskolin-stimulated PDE4D3 activity. Interestingly, PP2A holoenzyme associated with mAKAP complexes in the heart contains the PP2A targeting subunit B56delta. Like PDE4D3, B56delta is a PKA substrate, and PKA phosphorylation of mAKAP-bound B56delta enhances phosphatase activity 2-fold in the complex. Accordingly, expression of a B56delta mutant that cannot be phosphorylated by PKA results in increased PDE4D3 phosphorylation. Taken together, our findings demonstrate that PP2A associated with mAKAP complexes promotes PDE4D3 dephosphorylation, serving both to inhibit PDE4D3 in unstimulated cells and also to mediate a cAMP-induced positive feedback loop following adenylyl cyclase activation and B56delta phosphorylation. In general, PKA.PP2A.mAKAP complexes exemplify how protein kinases and phosphatases may participate in molecular signaling complexes to dynamically regulate localized intracellular signaling.
Figures
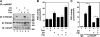






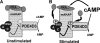
Similar articles
-
Protein kinase A and phosphodiesterase-4D3 binding to coding polymorphisms of cardiac muscle anchoring protein (mAKAP).J Mol Biol. 2013 Sep 23;425(18):3277-88. doi: 10.1016/j.jmb.2013.06.014. Epub 2013 Jun 25. J Mol Biol. 2013. PMID: 23806656 Free PMC article.
-
Human muscle-specific A-kinase anchoring protein polymorphisms modulate the susceptibility to cardiovascular diseases by altering cAMP/PKA signaling.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H109-H121. doi: 10.1152/ajpheart.00034.2018. Epub 2018 Mar 30. Am J Physiol Heart Circ Physiol. 2018. PMID: 29600899 Free PMC article.
-
mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module.EMBO J. 2001 Apr 17;20(8):1921-30. doi: 10.1093/emboj/20.8.1921. EMBO J. 2001. PMID: 11296225 Free PMC article.
-
The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways.Eur J Cell Biol. 2006 Jul;85(7):593-602. doi: 10.1016/j.ejcb.2006.01.007. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460834 Review.
-
Compartmentalization role of A-kinase anchoring proteins (AKAPs) in mediating protein kinase A (PKA) signaling and cardiomyocyte hypertrophy.Int J Mol Sci. 2014 Dec 24;16(1):218-29. doi: 10.3390/ijms16010218. Int J Mol Sci. 2014. PMID: 25547489 Free PMC article. Review.
Cited by
-
Targeting Odorant Receptors in Adipose Tissue with Food-Derived Odorants: A Novel Approach to Obesity Treatment.Foods. 2024 Dec 6;13(23):3938. doi: 10.3390/foods13233938. Foods. 2024. PMID: 39683011 Free PMC article. Review.
-
mAKAP-a master scaffold for cardiac remodeling.J Cardiovasc Pharmacol. 2015 Mar;65(3):218-25. doi: 10.1097/FJC.0000000000000206. J Cardiovasc Pharmacol. 2015. PMID: 25551320 Free PMC article. Review.
-
Renal AT2 Receptors Mediate Natriuresis via Protein Phosphatase PP2A.Circ Res. 2022 Jan 7;130(1):96-111. doi: 10.1161/CIRCRESAHA.121.319519. Epub 2021 Nov 19. Circ Res. 2022. PMID: 34794320 Free PMC article.
-
Unbiased Proteomic Profiling Uncovers a Targetable GNAS/PKA/PP2A Axis in Small Cell Lung Cancer Stem Cells.Cancer Cell. 2020 Jul 13;38(1):129-143.e7. doi: 10.1016/j.ccell.2020.05.003. Epub 2020 Jun 11. Cancer Cell. 2020. PMID: 32531271 Free PMC article.
-
cAMP signaling in subcellular compartments.Pharmacol Ther. 2014 Sep;143(3):295-304. doi: 10.1016/j.pharmthera.2014.03.008. Epub 2014 Apr 1. Pharmacol Ther. 2014. PMID: 24704321 Free PMC article. Review.
References
-
- Hayes J. S., Brunton L. L., Mayer S. E. (1980) J. Biol. Chem. 255, 5113–5119 - PubMed
-
- Brunton L. L., Hayes J. S., Mayer S. E. (1979) Nature 280, 78–80 - PubMed
-
- Keely S. L. (1977) Res. Commun. Chem. Pathol. Pharmacol. 18, 283–290 - PubMed
-
- Keely S. L. (1979) Mol. Pharmacol. 15, 235–245 - PubMed
-
- Steinberg S. F., Brunton L. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 751–773 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

