Targeting a novel N-terminal epitope of death receptor 5 triggers tumor cell death
- PMID: 20106985
- PMCID: PMC2838317
- DOI: 10.1074/jbc.M109.070680
Targeting a novel N-terminal epitope of death receptor 5 triggers tumor cell death
Abstract
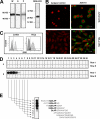
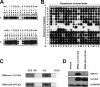
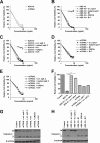
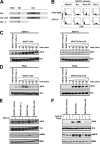
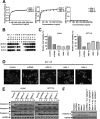
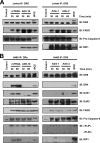
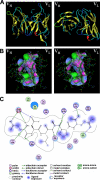
Tumor necrosis factor-related apoptosis-inducing ligand receptors death receptor (DR) 4 and DR5 are potential targets for antibody-based cancer therapy. Activation of the proapoptotic DR5 in various cancer cells triggers the extrinsic and/or intrinsic pathway of apoptosis. It has been shown that there are several functional domains in the DR5 extracellular domain. The cysteine-rich domains of DR5 have a conservative role in tumor necrosis factor-related apoptosis-inducing ligand-DR5-mediated apoptosis, and the pre-ligand assembly domain within the N1-cap contributes to the ligand-independent formation of receptor complexes. However, the role of the N-terminal region (NTR) preceding the N1-cap of DR5 remains unclear. In this study, we demonstrate that NTR could mediate DR5 activation that transmits an apoptotic signal when bound to a specific agonistic monoclonal antibody. A novel epitope in the NTR of DR5 was identified by peptide array. Antibodies against the antigenic determinant showed high affinities for DR5 and triggered caspase activation in a time-dependent manner, suggesting the NTR of DR5 might function as a potential death-inducing region. Moreover, permutation analysis showed that Leu(6) was pivotal for the interaction of DR5 and the agonistic antibody. Synthetic wild-type epitopes eliminated the cytotoxicity of all three agonistic monoclonal antibodies, AD5-10, Adie-1, and Adie-2. These results indicate that the NTR of DR5 could be a potential target site for the development of new strategies for cancer immunotherapy. Also, our findings expand the current knowledge about DR5 extracellular functional domains and provide insights into the mechanism of DR5-mediated cell death.
Figures
Similar articles
-
Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5.Cell Death Differ. 2008 Apr;15(4):751-61. doi: 10.1038/sj.cdd.4402306. Epub 2008 Jan 25. Cell Death Differ. 2008. PMID: 18219321
-
Synthetic ligands of death receptor 5 display a cell-selective agonistic effect at different oligomerization levels.Oncotarget. 2016 Oct 4;7(40):64942-64956. doi: 10.18632/oncotarget.10508. Oncotarget. 2016. PMID: 27409341 Free PMC article.
-
Structural and functional analysis of the N-terminal region of death receptor 5.Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011 Feb;33(1):33-8. doi: 10.3881/j.issn.1000-503X.2011.01.008. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011. PMID: 21375935
-
Cancer: novel therapeutic strategies that exploit the TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway.Int J Biochem Cell Biol. 2007;39(2):280-6. doi: 10.1016/j.biocel.2006.10.005. Epub 2006 Oct 7. Int J Biochem Cell Biol. 2007. PMID: 17097329 Review.
-
A novel agonistic anti-human death receptor 5 monoclonal antibody with tumoricidal activity induces caspase- and mitochondrial-dependent apoptosis in human leukemia Jurkat cells.Cancer Biother Radiopharm. 2011 Apr;26(2):143-52. doi: 10.1089/cbr.2010.0827. Cancer Biother Radiopharm. 2011. PMID: 21539448 Review.
Cited by
-
A novel anti-DR5 antibody-drug conjugate possesses a high-potential therapeutic efficacy for leukemia and solid tumors.Theranostics. 2019 Jul 13;9(18):5412-5423. doi: 10.7150/thno.33598. eCollection 2019. Theranostics. 2019. PMID: 31410224 Free PMC article.
-
The role of death effector domain-containing proteins in acute oxidative cell injury in hepatocytes.Free Radic Biol Med. 2012 May 1;52(9):1911-7. doi: 10.1016/j.freeradbiomed.2012.02.049. Epub 2012 Mar 8. Free Radic Biol Med. 2012. PMID: 22406316 Free PMC article.
-
DNA methylation profiling across the spectrum of HPV-associated anal squamous neoplasia.PLoS One. 2012;7(11):e50533. doi: 10.1371/journal.pone.0050533. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226306 Free PMC article.
-
2A peptide-based, lentivirus-mediated anti-death receptor 5 chimeric antibody expression prevents tumor growth in nude mice.Mol Ther. 2012 Jan;20(1):46-53. doi: 10.1038/mt.2011.197. Epub 2011 Sep 20. Mol Ther. 2012. PMID: 21934654 Free PMC article.
References
-
- Wiley S. R., Schooley K., Smolak P. J., Din W. S., Huang C. P., Nicholl J. K., Sutherland G. R., Smith T. D., Rauch C., Smith C. A., et al. (1995) Immunity 3, 673–682 - PubMed
-
- Pan G., O'Rourke K., Chinnaiyan A. M., Gentz R., Ebner R., Ni J., Dixit V. M. (1997) Science 276, 111–113 - PubMed
-
- Falschlehner C., Emmerich C. H., Gerlach B., Walczak H. (2007) Int. J. Biochem. Cell Biol. 39, 1462–1475 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

