Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure
- PMID: 20106993
- PMCID: PMC2853398
- DOI: 10.1152/ajpregu.00002.2010
Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure
Erratum in
- Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1448
Abstract
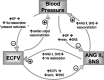
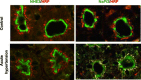
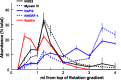
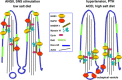
One-hundred years ago, Starling articulated the interdependence of renal control of circulating blood volume and effective cardiac performance. During the past 25 years, the molecular mechanisms responsible for the interdependence of blood pressure (BP), extracellular fluid volume (ECFV), the renin-angiotensin system (RAS), and sympathetic nervous system (SNS) have begun to be revealed. These variables all converge on regulation of renal proximal tubule (PT) sodium transport. The PT reabsorbs two-thirds of the filtered Na(+) and volume at baseline. This fraction is decreased when BP or perfusion pressure is increased, during a high-salt diet (elevated ECFV), and during inhibition of the production of ANG II; conversely, this fraction is increased by ANG II, SNS activation, and a low-salt diet. These variables all regulate the distribution of the Na(+)/H(+) exchanger isoform 3 (NHE3) and the Na(+)-phosphate cotransporter (NaPi2), along the apical microvilli of the PT. Natriuretic stimuli provoke the dynamic redistribution of these transporters along with associated regulators, molecular motors, and cytoskeleton-associated proteins to the base of the microvilli. The lipid raft-associated NHE3 remains at the base, and the nonraft-associated NaPi2 is endocytosed, culminating in decreased Na(+) transport and increased PT flow rate. Antinatriuretic stimuli return the same transporters and regulators to the body of the microvilli associated with an increase in transport activity and decrease in PT flow rate. In summary, ECFV and BP homeostasis are, at least in part, maintained by continuous and acute redistribution of transporter complexes up and down the PT microvilli, which affect regulation of PT sodium reabsorption in response to fluctuations in ECFV, BP, SNS, and RAS.
Figures

References
-
- Ahmed F, Kemp BA, Howell NL, Siragy HM, Carey RM. Extracellular renal guanosine cyclic 3'5'-monophosphate modulates nitric oxide and pressure-induced natriuresis. Hypertension 50: 958–963, 2007 - PubMed
-
- Azuma KK, Balkovetz DF, Magyar CE, Lescale-Matys L, Zhang Y, Chambrey R, Warnock DG, McDonough AA. Renal Na+/H+ exchanger isoforms and their regulation by thyroid hormone. Am J Physiol Cell Physiol 270: C585–C592, 1996 - PubMed
-
- Bianchi G. Genetic variations of tubular sodium reabsorption leading to “primary” hypertension: from gene polymorphism to clinical symptoms. Am J Physiol Regul Integr Comp Physiol 289: R1536–R1549, 2005 - PubMed
-
- Biemesderfer D, Mentone SA, Mooseker M, Hasson T. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am J Physiol Renal Physiol 282: F785–F794, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

