In situ immunolocalization and stage-dependent expression of a secretory serine protease in Leishmania donovani and its role as a vaccine candidate
- PMID: 20106998
- PMCID: PMC2849349
- DOI: 10.1128/CVI.00358-09
In situ immunolocalization and stage-dependent expression of a secretory serine protease in Leishmania donovani and its role as a vaccine candidate
Abstract
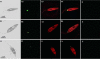
Proteases have been found to play essential roles in many biological processes, including the pathogenesis of leishmaniasis. Most parasites rely on their intracellular and extracellular protease repertoire to invade and multiply in mammalian host cells. However, few studies have addressed serine proteases in Leishmania and their role in host pathogenesis. Here we report the intracellular distribution of a novel L. donovani secretory serine protease in the flagellar pocket, as determined by immunogold labeling. Flow cytometry and confocal immunofluorescence analysis revealed that the expression of the protease diminishes sequentially from virulent to attenuated strains of this species and is also highly associated with the metacyclic stage of L. donovani promastigotes. The level of internalization of parasites treated with the anti-115-kDa antibody into host macrophages was significantly reduced from that of non-antibody-treated parasites, suggesting that this serine protease probably plays a role in the infection process. In vivo studies confirmed that this serine protease is a potential vaccine candidate. Altogether, the 115-kDa serine protease might play vital roles in L. donovani pathogenesis and hence could be recognized as a potential candidate for drug design.
Figures
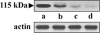


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

