Coincorporation of LpxL1 and PagL mutant lipopolysaccharides into liposomes with Neisseria meningitidis opacity protein: influence on endotoxic and adjuvant activity
- PMID: 20107001
- PMCID: PMC2849350
- DOI: 10.1128/CVI.00423-09
Coincorporation of LpxL1 and PagL mutant lipopolysaccharides into liposomes with Neisseria meningitidis opacity protein: influence on endotoxic and adjuvant activity
Abstract
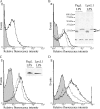
Wild-type lipopolysaccharide (LPS) of Neisseria meningitidis normally contains six acyl chains. Penta-acylated LPS forms were generated through inactivation of the lpxL1 gene or through the expression of the Bordetella bronchiseptica pagL gene in N. meningitidis. The resulting LPS species, designated LpxL1 LPS and PagL LPS, respectively, display reduced endotoxic activity compared to wild-type LPS. Here, we determined the adjuvant potential of PagL LPS by comparison with the broadly used LpxL1 LPS. We also investigated the potential benefit for adjuvanticity of coincorporating these LPS species, together with the meningococcal opacity-associated protein OpaJ as a model antigen, in a liposomal delivery system. PagL LPS showed a higher endotoxic activity than LpxL1 LPS, and their incorporation into liposomes significantly reduced their endotoxic activity as determined by measuring the induction of interleukin-6 (IL-6) production in a murine macrophage cell line. To determine the adjuvant effect, BALB/c mice were immunized with OpaJ-containing liposomes and either free LPS or LPS coincorporated into the proteoliposomes. OpaJ-containing liposomes adjuvanted with AlPO(4) or not adjuvanted at all were included as control groups. In the appropriate dose, PagL LPS showed a superior adjuvant effect compared with LpxL1 LPS, and for both LPS types, free LPS showed a higher adjuvant effect than when coincorporated into the liposomes, as evidenced by higher titers of IgG2a and IgG2b antibodies against OpaJ(+) meningococci and higher bactericidal titers. In conclusion, PagL LPS is a better adjuvant than LpxL1 LPS, but coincorporation of either LPS into proteoliposomes did not improve their adjuvant activity.
Figures
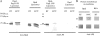


Similar articles
-
Lipopolysaccharide engineering in Neisseria meningitidis: structural analysis of different pentaacyl lipid A mutants and comparison of their modified agonist properties.J Biol Chem. 2014 Mar 21;289(12):8668-80. doi: 10.1074/jbc.M114.554345. Epub 2014 Feb 3. J Biol Chem. 2014. PMID: 24492609 Free PMC article.
-
A critical contribution of both CD28 and ICOS in the adjuvant activity of Neisseria meningitidis H44/76 LPS and lpxL1 LPS.Vaccine. 2007 Jun 11;25(24):4681-8. doi: 10.1016/j.vaccine.2007.04.016. Epub 2007 Apr 25. Vaccine. 2007. PMID: 17499399
-
Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives.Vaccine. 2005 Oct 17;23(43):5091-8. doi: 10.1016/j.vaccine.2005.06.001. Vaccine. 2005. PMID: 15993990
-
Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines.Expert Rev Vaccines. 2011 Mar;10(3):323-34. doi: 10.1586/erv.11.10. Expert Rev Vaccines. 2011. PMID: 21434800 Review.
-
Meningococcal lipopolysaccharides: virulence factor and potential vaccine component.Microbiol Rev. 1993 Mar;57(1):34-49. doi: 10.1128/mr.57.1.34-49.1993. Microbiol Rev. 1993. PMID: 8464406 Free PMC article. Review.
Cited by
-
Meningococcal Vaccines: Current Status and Emerging Strategies.Vaccines (Basel). 2018 Feb 25;6(1):12. doi: 10.3390/vaccines6010012. Vaccines (Basel). 2018. PMID: 29495347 Free PMC article. Review.
-
Improvement of immunogenicity of meningococcal lipooligosaccharide by coformulation with lipidated transferrin-binding protein B in liposomes: implications for vaccine development.Clin Vaccine Immunol. 2012 May;19(5):711-22. doi: 10.1128/CVI.05683-11. Epub 2012 Mar 21. Clin Vaccine Immunol. 2012. PMID: 22441387 Free PMC article.
-
Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains.Vaccine. 2013 Feb 18;31(9):1298-304. doi: 10.1016/j.vaccine.2012.12.076. Epub 2013 Jan 9. Vaccine. 2013. PMID: 23313617 Free PMC article.
-
Microbial biosynthesis of designer outer membrane vesicles.Curr Opin Biotechnol. 2014 Oct;29:76-84. doi: 10.1016/j.copbio.2014.02.018. Epub 2014 Mar 22. Curr Opin Biotechnol. 2014. PMID: 24667098 Free PMC article. Review.
-
Bacterial outer membrane vesicle-based cancer nanovaccines.Cancer Biol Med. 2022 Sep 23;19(9):1290-300. doi: 10.20892/j.issn.2095-3941.2022.0452. Cancer Biol Med. 2022. PMID: 36172794 Free PMC article. Review.
References
-
- Arenas, J., A. Abel, S. Sánchez, J. Marzoa, S. Berrón, P. van der Ley, M. T. Criado, and C. M. Ferreiros. 2008. A cross-reactive neisserial antigen encoded by the NMB0035 locus shows high sequence conservation but variable surface accessibility. J. Med. Microbiol. 57:80-87. - PubMed
-
- Arigita, C., W. Jiskoot, J. Westdijk, C. van Ingen, W. E. Hennink, D. J. A. Crommelin, and G. F. A. Kersten. 2004. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 22:629-642. - PubMed
-
- Arigita, C., T. Luijkx, W. Jiskoot, M. Poelen, W. E. Hennink, D. J. A. Crommelin, P. van der Ley, C. van Els, and G. F. A. Kersten. 2005. Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives. Vaccine 23:5091-5098. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

