Dexamethasone stimulates store-operated calcium entry and protein degradation in cultured L6 myotubes through a phospholipase A(2)-dependent mechanism
- PMID: 20107037
- PMCID: PMC2867385
- DOI: 10.1152/ajpcell.00309.2009
Dexamethasone stimulates store-operated calcium entry and protein degradation in cultured L6 myotubes through a phospholipase A(2)-dependent mechanism
Abstract
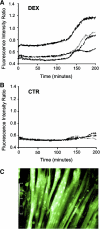
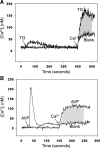
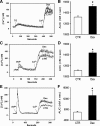
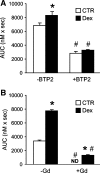
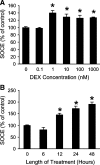
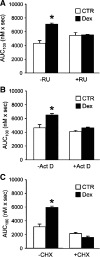
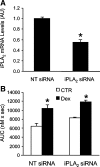
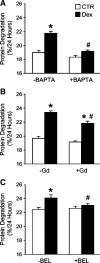
Muscle wasting in various catabolic conditions is at least in part regulated by glucocorticoids. Increased calcium levels have been reported in atrophying muscle. Mechanisms regulating calcium homeostasis in muscle wasting, in particular the role of glucocorticoids, are poorly understood. Here we tested the hypothesis that glucocorticoids increase intracellular calcium concentrations in skeletal muscle and stimulate store-operated calcium entry (SOCE) and that these effects of glucocorticoids may at least in part be responsible for glucocorticoid-induced protein degradation. Treatment of cultured myotubes with dexamethasone, a frequently used in vitro model of muscle wasting, resulted in increased intracellular calcium concentrations determined by fura-2 AM fluorescence measurements. When SOCE was measured by using calcium "add-back" to muscle cells after depletion of intracellular calcium stores, results showed that SOCE was increased 15-25% by dexamethasone and that this response to dexamethasone was inhibited by the store-operated calcium channel blocker BTP2. Dexamethasone treatment stimulated the activity of calcium-independent phospholipase A(2) (iPLA(2)), and dexamethasone-induced increase in SOCE was reduced by the iPLA(2) inhibitor bromoenol lactone (BEL). In additional experiments, treatment of myotubes with the store-operated calcium channel inhibitor gadolinium ion or BEL reduced dexamethasone-induced increase in protein degradation. Taken together, the results suggest that glucocorticoids increase calcium concentrations in myocytes and stimulate iPLA(2)-dependent SOCE and that glucocorticoid-induced muscle protein degradation may at least in part be regulated by increased iPLA(2) activity, SOCE, and cellular calcium levels.
Figures

Similar articles
-
Calcium-independent phospholipase A2 mediates store-operated calcium entry in rat cerebellar granule cells.Cerebellum. 2008;7(3):467-81. doi: 10.1007/s12311-008-0050-z. Cerebellum. 2008. PMID: 18784973
-
Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes.J Cell Biochem. 2008 Oct 1;105(2):353-64. doi: 10.1002/jcb.21833. J Cell Biochem. 2008. PMID: 18615595 Free PMC article.
-
Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent.Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R337-4. doi: 10.1152/ajpregu.00230.2006. Epub 2006 Sep 14. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 16973938
-
Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes.J Neurosci. 2006 Sep 13;26(37):9579-92. doi: 10.1523/JNEUROSCI.2604-06.2006. J Neurosci. 2006. PMID: 16971542 Free PMC article.
-
Phospholipase A2 as a Molecular Determinant of Store-Operated Calcium Entry.Adv Exp Med Biol. 2016;898:111-31. doi: 10.1007/978-3-319-26974-0_6. Adv Exp Med Biol. 2016. PMID: 27161227 Review.
Cited by
-
In vivo effects of dexamethasone on blood gene expression in ataxia telangiectasia.Mol Cell Biochem. 2018 Jan;438(1-2):153-166. doi: 10.1007/s11010-017-3122-x. Epub 2017 Jul 25. Mol Cell Biochem. 2018. PMID: 28744812 Clinical Trial.
-
Severe disturbance in the Ca2+ signaling in astrocytes from mouse models of human infantile neuroaxonal dystrophy with mutated Pla2g6.Hum Mol Genet. 2012 Jun 15;21(12):2807-14. doi: 10.1093/hmg/dds108. Epub 2012 Mar 22. Hum Mol Genet. 2012. PMID: 22442204 Free PMC article.
-
Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy.J Neuromuscul Dis. 2021;8(1):39-52. doi: 10.3233/JND-200556. J Neuromuscul Dis. 2021. PMID: 33104035 Free PMC article. Review.
-
Loss of muscle strength during sepsis is in part regulated by glucocorticoids and is associated with reduced muscle fiber stiffness.Am J Physiol Regul Integr Comp Physiol. 2012 Nov 15;303(10):R1090-9. doi: 10.1152/ajpregu.00636.2011. Epub 2012 Sep 26. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23019215 Free PMC article.
-
Long-term dexamethasone treatment diminishes store-operated Ca2+ entry in salivary acinar cells.Int J Oral Sci. 2019 Jan 3;11(1):1. doi: 10.1038/s41368-018-0031-0. Int J Oral Sci. 2019. PMID: 30602784 Free PMC article.
References
-
- Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem 270: 445–450, 1995 - PubMed
-
- Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275: 9452–9460, 2000 - PubMed
-
- Basset O, Boittin FX, Dorchies OM, Chatton JY, van Breemen C, Ruegg UT. Involvement of 1,4,5-triphosphate in nicotinic calcium responses in dystrophic myotubes assessed by near-plasma membrane calcium measurement. J Biol Chem 279: 47092–47100, 2004 - PubMed
-
- Benson DW, Hasselgren PO, Hiyama DT, James JH, Li S, Rigel DF, Fischer JE. Effect of sepsis on calcium uptake and content in skeletal muscle and regulation in vitro by calcium of total and myofibrillar protein breakdown in control and septic muscle: results from a preliminary study. Surgery 106: 87–93, 1989 - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Ca2+ signaling: dynamics, homeostasis, remodeling. Nat Rev Mol Cell Biol 4: 517–529, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

