Collagen I matrix turnover is regulated by fibronectin polymerization
- PMID: 20107040
- PMCID: PMC2867395
- DOI: 10.1152/ajpcell.00341.2009
Collagen I matrix turnover is regulated by fibronectin polymerization
Abstract
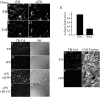
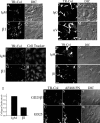
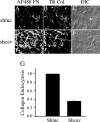
Extracellular matrix (ECM) remodeling occurs during normal homeostasis and also plays an important role during development, tissue repair, and in various disease processes. ECM remodeling involves changes in the synthesis, deposition, and degradation of ECM molecules. ECM molecules can be degraded extracellularly, as well as intracellularly following endocytosis. Our data show that the ECM protein fibronectin is an important regulator of ECM remodeling. We previously showed that agents that inhibit the polymerization of fibronectin into ECM fibrils promote the loss of preexisting fibronectin matrix and accelerate fibronectin endocytosis and degradation. In this paper we show that inhibition of fibronectin polymerization leads to the loss of collagen I matrix fibrils and a corresponding increase in the levels of endocytosed collagen I. In contrast, manipulations that stabilize fibronectin matrix fibrils, such as caveolin-1 depletion, stabilize collagen I matrix fibrils and cause a decrease in ECM collagen I endocytosis. Our data also show that endocytosis of ECM collagen I is regulated by both beta1 integrins and Endo180/urokinase plasminogen activator associated protein (uPARAP). Unexpectedly, Endo180/uPARAP was also shown to promote the endocytosis of fibronectin from the ECM. These data demonstrate that fibronectin polymerization regulates the remodeling of ECM collagen I, in part, by regulating collagen I endocytosis. Furthermore, these data show that processes that regulate ECM deposition coordinately regulate the removal of proteins from the ECM. These data highlight the complexity of ECM remodeling. This multifaceted regulatory process may be important to ensure tight regulation of ECM fibronectin and collagen I levels.
Figures




References
-
- Ala-aho R, Kahari VM. Collagenases in cancer. Biochimie 87: 273–286, 2005 - PubMed
-
- Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem 279: 35749–35759, 2004 - PubMed
-
- Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum 29: 252–265, 2000 - PubMed
-
- Bhide VM, Laschinger CA, Arora PD, Lee W, Hakkinen L, Larjava H, Sodek J, McCulloch CA. Collagen phagocytosis by fibroblasts is regulated by decorin. J Biol Chem 280: 23103–23113, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

