Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods
- PMID: 20107049
- PMCID: PMC2824328
- DOI: 10.1523/JNEUROSCI.4353-09.2010
Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods
Abstract
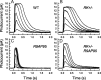
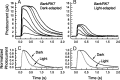
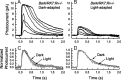
The Ca(2+)-binding protein recoverin is thought to regulate rhodopsin kinase and to modulate the lifetime of the photoexcited state of rhodopsin (Rh*), the visual pigment of vertebrate rods. Recoverin has been postulated to inhibit the kinase in darkness, when Ca(2+) is high, and to be released from the disk membrane in light when Ca(2+) is low, accelerating rhodopsin phosphorylation and shortening the lifetime of Rh*. This proposal has remained controversial, in part because the normally rapid turnoff of Rh* has made Rh* modulation difficult to study in an intact rod. To circumvent this problem, we have made mice that underexpress rhodopsin kinase so that Rh* turnoff is rate limiting for the decay of the rod light response. We show that background light speeds the decay of Rh* turnoff, and that this no longer occurs in mice that have had recoverin knocked out. This is the first demonstration in an intact rod that light accelerates Rh* inactivation and that the Ca(2+)-binding protein recoverin may be required for the light-dependent modulation of Rh* lifetime.
Figures





Similar articles
-
Modulation of mouse rod response decay by rhodopsin kinase and recoverin.J Neurosci. 2012 Nov 7;32(45):15998-6006. doi: 10.1523/JNEUROSCI.1639-12.2012. J Neurosci. 2012. PMID: 23136436 Free PMC article.
-
Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation.J Gen Physiol. 2015 Mar;145(3):213-24. doi: 10.1085/jgp.201411273. Epub 2015 Feb 9. J Gen Physiol. 2015. PMID: 25667411 Free PMC article.
-
Mechanism of rhodopsin kinase regulation by recoverin.J Neurochem. 2009 Jul;110(1):72-9. doi: 10.1111/j.1471-4159.2009.06118.x. Epub 2009 Apr 27. J Neurochem. 2009. PMID: 19457073
-
Control of rhodopsin activity in vision.Eye (Lond). 1998;12 ( Pt 3b):521-5. doi: 10.1038/eye.1998.140. Eye (Lond). 1998. PMID: 9775212 Review.
-
Calcium-dependent regulation of rhodopsin phosphorylation.Novartis Found Symp. 1999;224:208-18; discussion 218-24. doi: 10.1002/9780470515693.ch12. Novartis Found Symp. 1999. PMID: 10614053 Review.
Cited by
-
C-terminal threonines and serines play distinct roles in the desensitization of rhodopsin, a G protein-coupled receptor.Elife. 2015 Apr 24;4:e05981. doi: 10.7554/eLife.05981. Elife. 2015. PMID: 25910054 Free PMC article.
-
A highly conserved cysteine of neuronal calcium-sensing proteins controls cooperative binding of Ca2+ to recoverin.J Biol Chem. 2013 Dec 13;288(50):36160-7. doi: 10.1074/jbc.M113.524355. Epub 2013 Nov 4. J Biol Chem. 2013. PMID: 24189072 Free PMC article.
-
Discovering electrophysiology in photobiology: A brief overview of several photobiological processes with an emphasis on electrophysiology.Commun Integr Biol. 2014 Mar 12;7:e28423. doi: 10.4161/cib.28423. eCollection 2014. Commun Integr Biol. 2014. PMID: 25328636 Free PMC article. Review.
-
Spatiotemporal cGMP dynamics in living mouse rods.Biophys J. 2012 Apr 18;102(8):1775-84. doi: 10.1016/j.bpj.2012.03.035. Biophys J. 2012. PMID: 22768933 Free PMC article.
-
Voltage-clamp recordings of light responses from wild-type and mutant mouse cone photoreceptors.J Gen Physiol. 2019 Nov 4;151(11):1287-1299. doi: 10.1085/jgp.201912419. Epub 2019 Sep 27. J Gen Physiol. 2019. PMID: 31562185 Free PMC article.
References
-
- Ames JB, Levay K, Wingard JN, Lusin JD, Slepak VZ. Structural basis for calcium-induced inhibition of rhodopsin kinase by recoverin. J Biol Chem. 2006;281:37237–37245. - PubMed
-
- Chen C-K, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. - PubMed
-
- Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 1995;267:374–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
