A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia
- PMID: 20107055
- PMCID: PMC6633802
- DOI: 10.1523/JNEUROSCI.5408-09.2010
A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia
Abstract
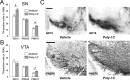
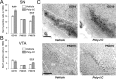
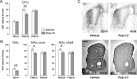
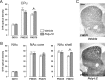
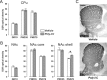
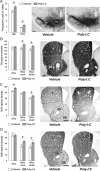
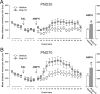
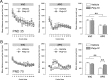
Prenatal exposure to infection is a significant environmental risk factor in the development of schizophrenia and related disorders. Recent evidence indicates that disruption of functional and structural dopaminergic development may be at the core of the developmental neuropathology associated with psychosis-related abnormalities induced by prenatal exposure to infection. Using a mouse model of prenatal immune challenge by the viral mimic polyriboinosinic-polyribocytidilic acid, the present study critically evaluated this hypothesis by longitudinally monitoring the effects of maternal immune challenge during pregnancy on structural and functional dopaminergic development in the offspring from fetal to adult stages of life. Our study shows that prenatal immune challenge leads to dopaminergic maldevelopment starting as early as in the fetal stages of life and produces a set of postnatal dopaminergic abnormalities that is dependent on postnatal maturational processes. Furthermore, our longitudinal investigations reveal a striking developmental correspondence between the ontogeny of specific dopaminergic neuropathology and the postnatal onset of distinct forms of dopamine-dependent functional abnormalities implicated in schizophrenia. Prenatal immune activation thus appears to be a significant environmental risk factor for primary defects in normal dopaminergic development and facilitates the expression of postnatal dopamine dysfunctions involved in the precipitation of psychosis-related behavior. Early interventions targeting the developing dopamine system may open new avenues for a successful attenuation or even prevention of psychotic disorders following neurodevelopmental disruption of dopamine functions.
Figures

Similar articles
-
Neural basis of psychosis-related behaviour in the infection model of schizophrenia.Behav Brain Res. 2009 Dec 7;204(2):322-34. doi: 10.1016/j.bbr.2008.12.022. Epub 2008 Dec 30. Behav Brain Res. 2009. PMID: 19154759
-
Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia.Psychopharmacology (Berl). 2009 Nov;206(4):587-602. doi: 10.1007/s00213-009-1504-9. Epub 2009 Mar 11. Psychopharmacology (Berl). 2009. PMID: 19277608 Review.
-
Nurr1 is not essential for the development of prepulse inhibition deficits induced by prenatal immune activation.Brain Behav Immun. 2011 Oct;25(7):1316-21. doi: 10.1016/j.bbi.2011.06.012. Epub 2011 Jun 24. Brain Behav Immun. 2011. PMID: 21723940
-
Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia.Int J Neuropsychopharmacol. 2009 May;12(4):513-24. doi: 10.1017/S1461145708009206. Epub 2008 Aug 28. Int J Neuropsychopharmacol. 2009. PMID: 18752727
-
Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models.Transl Psychiatry. 2012 Feb 21;2(2):e81. doi: 10.1038/tp.2012.6. Transl Psychiatry. 2012. PMID: 22832818 Free PMC article. Review.
Cited by
-
Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome.Schizophr Bull. 2014 Mar;40(2):351-61. doi: 10.1093/schbul/sbs195. Epub 2013 Jan 17. Schizophr Bull. 2014. PMID: 23328159 Free PMC article.
-
Advantages and Limitations of Animal Schizophrenia Models.Int J Mol Sci. 2022 May 25;23(11):5968. doi: 10.3390/ijms23115968. Int J Mol Sci. 2022. PMID: 35682647 Free PMC article. Review.
-
Clonal Characteristics of T-Cell Receptor Repertoires in Violent and Non-violent Patients With Schizophrenia.Front Psychiatry. 2018 Aug 31;9:403. doi: 10.3389/fpsyt.2018.00403. eCollection 2018. Front Psychiatry. 2018. PMID: 30233426 Free PMC article.
-
Preliminary evidence of increased striatal dopamine in a nonhuman primate model of maternal immune activation.Transl Psychiatry. 2019 Apr 12;9(1):135. doi: 10.1038/s41398-019-0449-y. Transl Psychiatry. 2019. PMID: 30979867 Free PMC article.
-
Sensory filtering disruption caused by poly I:C - Timing of exposure and other experimental considerations.Brain Behav Immun Health. 2020 Oct 8;9:100156. doi: 10.1016/j.bbih.2020.100156. eCollection 2020 Dec. Brain Behav Immun Health. 2020. PMID: 34589898 Free PMC article.
References
-
- Bacopoulos NG, Bhatnagar RK. Correlation between tyrosine hydroxylase activity and catecholamine concentration or turnover in brain regions. J Neurochem. 1977;29:639–643. - PubMed
-
- Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. - PubMed
-
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;6:204–221. - PubMed
-
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
