Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission
- PMID: 20107062
- PMCID: PMC2823378
- DOI: 10.1523/JNEUROSCI.3427-09.2010
Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission
Abstract
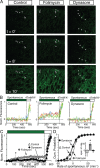
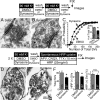
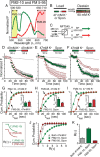
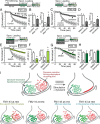
Synapses maintain synchronous, asynchronous, and spontaneous forms of neurotransmission that are distinguished by their Ca(2+) dependence and time course. Despite recent advances in our understanding of the mechanisms that underlie these three forms of release, it remains unclear whether they originate from the same vesicle population or arise from distinct vesicle pools with diverse propensities for release. Here, we used a reversible inhibitor of dynamin, dynasore, to dissect the vesicle pool dynamics underlying the three forms of neurotransmitter release in hippocampal GABAergic inhibitory synapses. In dynasore, evoked synchronous release and asynchronous neurotransmission detected after activity showed marked and unrecoverable depression within seconds. In contrast, spontaneous release remained intact after intense stimulation in dynasore or during prolonged (approximately 1 h) application of dynasore at rest, suggesting that separate recycling pathways maintain evoked and spontaneous synaptic vesicle trafficking. In addition, simultaneous imaging of spectrally separable styryl dyes revealed that, in a given synapse, vesicles that recycle spontaneously and in response to activity do not mix. These findings suggest that evoked synchronous and asynchronous release originate from the same vesicle pool that recycles rapidly in a dynamin-dependent manner, whereas a distinct vesicle pool sustains spontaneous release independent of dynamin activation. This result lends additional support to the notion that synapses harbor distinct vesicle populations with divergent release properties that maintain independent forms of neurotransmission.
Figures





Comment in
-
Parsing spontaneous and evoked neurotransmission on both sides of the synapse.J Neurosci. 2010 May 12;30(19):6480-1. doi: 10.1523/JNEUROSCI.1177-10.2010. J Neurosci. 2010. PMID: 20463211 Free PMC article. No abstract available.
References
-
- Barylko B, Binns D, Lin KM, Atkinson MA, Jameson DM, Yin HL, Albanesi JP. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J Biol Chem. 1998;273:3791–3797. - PubMed
-
- Cremona O, De Camilli P. Synaptic vesicle endocytosis. Curr Opin Neurobiol. 1997;7:323–330. - PubMed
-
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
