The Sushi domains of GABAB receptors function as axonal targeting signals
- PMID: 20107064
- PMCID: PMC6633810
- DOI: 10.1523/JNEUROSCI.3172-09.2010
The Sushi domains of GABAB receptors function as axonal targeting signals
Abstract
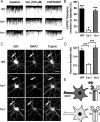
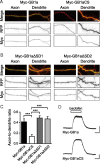
GABA(B) receptors are the G-protein-coupled receptors for GABA, the main inhibitory neurotransmitter in the brain. Two receptor subtypes, GABA(B(1a,2)) and GABA(B(1b,2)), are formed by the assembly of GABA(B1a) and GABA(B1b) subunits with GABA(B2) subunits. The GABA(B1b) subunit is a shorter isoform of the GABA(B1a) subunit lacking two N-terminal protein interaction motifs, the sushi domains. Selectively GABA(B1a) protein traffics into the axons of glutamatergic neurons, whereas both the GABA(B1a) and GABA(B1b) proteins traffic into the dendrites. The mechanism(s) and targeting signal(s) responsible for the selective trafficking of GABA(B1a) protein into axons are unknown. Here, we provide evidence that the sushi domains are axonal targeting signals that redirect GABA(B1a) protein from its default dendritic localization to axons. Specifically, we show that mutations in the sushi domains preventing protein interactions preclude axonal localization of GABA(B1a). When fused to CD8alpha, the sushi domains polarize this uniformly distributed protein to axons. Likewise, when fused to mGluR1a the sushi domains redirect this somatodendritic protein to axons, showing that the sushi domains can override dendritic targeting information in a heterologous protein. Cell surface expression of the sushi domains is not required for axonal localization of GABA(B1a). Altogether, our findings are consistent with the sushi domains functioning as axonal targeting signals by interacting with axonally bound proteins along intracellular sorting pathways. Our data provide a mechanistic explanation for the selective trafficking of GABA(B(1a,2)) receptors into axons while at the same time identifying a well defined axonal delivery module that can be used as an experimental tool.
Figures




References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel JM, Pin JP, Bockaert J, Fagni L. A simple method to transfer plasmid DNA into neuronal primary cultures: functional expression of the mGlu5 receptor in cerebellar granule cells. Neuropharmacology. 1999;38:793–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
