Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula
- PMID: 20107084
- PMCID: PMC6633804
- DOI: 10.1523/JNEUROSCI.3690-09.2010
Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula
Abstract
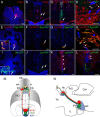
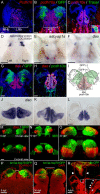
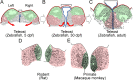
The mammalian habenula consists of the medial and lateral habenulae. Recent behavioral and electrophysiological studies suggested that the lateral habenula plays a pivotal role in controlling motor and cognitive behaviors by influencing the activity of dopaminergic and serotonergic neurons. Despite the functional significance, manipulating neural activity in this pathway remains difficult because of the absence of a genetically accessible animal model such as zebrafish. To address the level of lateral habenula conservation in zebrafish, we applied the tract-tracing technique to GFP (green fluorescent protein)-expressing transgenic zebrafish to identify habenular neurons that project to the raphe nuclei, a major target of the mammalian lateral habenula. Axonal tracing in live and fixed fish showed projection of zebrafish ventral habenula axons to the ventral part of the median raphe, but not to the interpeduncular nucleus where the dorsal habenula projected. The ventral habenula expressed protocadherin 10a, a specific marker of the rat lateral habenula, whereas the dorsal habenula showed no such expression. Gene expression analyses revealed that the ventromedially positioned ventral habenula in the adult originated from the region of primordium lateral to the dorsal habenula during development. This suggested that zebrafish habenulae emerge during development with mediolateral orientation similar to that of the mammalian medial and lateral habenulae. These findings indicated that the lateral habenular pathways are evolutionarily conserved pathways and might control adaptive behaviors in vertebrates through the regulation of monoaminergic activities.
Figures

References
-
- Aizawa H, Goto M, Sato T, Okamoto H. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12:87–98. - PubMed
-
- Allen Institute for Brain Science. Seattle: Allen Institute for Brain Science; 2009. Allen mouse brain atlas. Available at http://mouse.brain-map.org.
-
- Almeida AP, Beaven MA. Phylogeny of histamine in vertebrate brain. Brain Res. 1981;208:244–250. - PubMed
-
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
