Analytical performance characteristics and clinical utility of a novel assay for total hepatitis C virus core antigen quantification
- PMID: 20107102
- PMCID: PMC2849592
- DOI: 10.1128/JCM.01640-09
Analytical performance characteristics and clinical utility of a novel assay for total hepatitis C virus core antigen quantification
Abstract
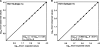
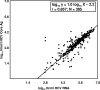
The detection and quantification of hepatitis C virus (HCV) core antigen in serum or plasma by the use of different assay formats have previously been shown to represent useful markers of viral replication. In the present study, the intrinsic performance characteristics and the potential clinical utility of a novel assay for the quantification of total HCV core antigen were comprehensively assessed by using clinical serum samples and specimens contained in various evaluation panels. The Architect HCV Ag assay showed a specificity of 100%. The intra- and interassay coefficients of variation ranged from 3.6 to 8.0% and from 4.7 to 9.5%, respectively. Except for HCV genotype 2 isolates, the analytical sensitivity was always less than 10 fmol core antigen/liter, corresponding to approximately 500 to 3,000 IU of HCV RNA/ml. Linearity was guaranteed throughout the dynamic range (10 to 20,000 fmol/liter). When seroconversion panels were tested, the assay was not inferior to HCV RNA detection and reduced the preseroconversion period by 4 to 16 days. The results obtained by core antigen and HCV RNA quantification for 385 clinical specimens were correlated by regression analysis (r = 0.857), but the calculated conversion equation differed significantly from the line of identity. Monitoring of viral kinetics by use of either core antigen or RNA concentrations in 38 HCV-infected patients undergoing antiviral combination therapy resulted in very similarly shaped curves in all cases. Finally, the Architect HCV Ag assay was also shown to enable high-throughput screening of in vitro HCV RNA replication. With these results taken together, the Architect HCV Ag assay proved to be a specific, reproducible, highly sensitive, and clinically applicable test format which will find its future place in the context of virological HCV diagnostics.
Figures

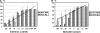
References
-
- Bablok, W. 1993. Range of linearity, p. 251-258. In R. Haeckel (ed.), Evaluation methods in laboratory medicine. VHC, Weinheim, Germany.
-
- Blatt, L. M., M. G. Mutchnik, M. J. Tong, F. M. Klion, E. Lebovics, B. Freilich, N. Bach, C. Smith, J. Herrera, H. Tobias, A. Conrad, P. Schmid, and J. G. McHutchinson. 2000. Assessment of hepatitis C virus RNA and genotype from 6807 patients with chronic hepatitis C in the United States. J. Viral Hepat. 7:196-202. - PubMed
-
- Bouvier-Alias, M., K. Patel, H. Dahari, S. Beaucourt, P. Larderie, L. Blatt, C. Hezode, G. Picchio, D. Dhumeaux, A. U. Neumann, J. G. McHutchinson, and J.-M. Pawlotsky. 2002. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36:211-218. - PubMed
-
- Bouzgarrou, N., I. Fodha, S. Ben Othman, A. Achour, F. Grattard, A. Trabelsi, and B. Pozzetto. 2005. Evaluation of a total core antigen assay for the diagnosis of hepatitis C virus infection in hemodialysis patients. J. Med. Virol. 77:502-508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

