Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin
- PMID: 20107105
- PMCID: PMC2858197
- DOI: 10.2337/dc09-1521
Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin
Abstract
Objective: Study the effects of exenatide (EXE) plus rosiglitazone (ROSI) on beta-cell function and insulin sensitivity using hyperglycemic and euglycemic insulin clamp techniques in participants with type 2 diabetes on metformin.
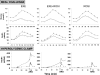
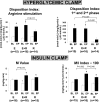
Research design and methods: In this 20-week, randomized, open-label, multicenter study, participants (mean age, 56 +/- 10 years; weight, 93 +/- 16 kg; A1C, 7.8 +/- 0.7%) continued their metformin regimen and received either EXE 10 microg b.i.d. (n = 45), ROSI 4 mg b.i.d. (n = 45), or EXE 10 microg b.i.d. + ROSI 4 mg b.i.d. (n = 47). Seventy-three participants underwent clamp procedures to quantitate insulin secretion and insulin sensitivity. RESULTS A1C declined in all groups (P < 0.05), but decreased most with EXE+ROSI (EXE+ROSI, -1.3 +/- 0.1%; ROSI, -1.0 +/- 0.1%, EXE, -0.9 +/- 0.1%; EXE+ROSI vs. EXE or ROSI, P < 0.05). ROSI resulted in weight gain, while EXE and EXE+ROSI resulted in weight loss (EXE, -2.8 +/- 0.5 kg; EXE+ROSI, -1.2 +/- 0.5 kg; ROSI, + 1.5 +/- 0.5 kg; P < 0.05 between and within all groups). At week 20, 1st and 2nd phase insulin secretion was significantly higher in EXE and EXE+ROSI versus ROSI (both P < 0.05). Insulin sensitivity (M value) was significantly higher in EXE+ROSI versus EXE (P = 0.014).
Conclusions: Therapy with EXE+ROSI offset the weight gain observed with ROSI and elicited an additive effect on glycemic control with significant improvements in beta-cell function and insulin sensitivity.
Trial registration: ClinicalTrials.gov NCT00135330.
Figures
Similar articles
-
Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study.Diabetes Care. 2010 Jul;33(7):1509-15. doi: 10.2337/dc09-2191. Epub 2010 Mar 31. Diabetes Care. 2010. PMID: 20357372 Free PMC article. Clinical Trial.
-
Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea.Diabetes Care. 2005 May;28(5):1083-91. doi: 10.2337/diacare.28.5.1083. Diabetes Care. 2005. PMID: 15855571 Clinical Trial.
-
Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes.Diabetes Care. 2011 Sep;34(9):2041-7. doi: 10.2337/dc11-0291. Diabetes Care. 2011. PMID: 21868779 Free PMC article. Clinical Trial.
-
Targeting the pathophysiology of type 2 diabetes: rationale for combination therapy with pioglitazone and exenatide.Curr Med Res Opin. 2008 Nov;24(11):3009-22. doi: 10.1185/03007990802390795. Epub 2008 Oct 2. Curr Med Res Opin. 2008. PMID: 18828960 Review.
-
Insulin Release Mechanism Modulated by Toxins Isolated from Animal Venoms: From Basic Research to Drug Development Prospects.Molecules. 2019 May 14;24(10):1846. doi: 10.3390/molecules24101846. Molecules. 2019. PMID: 31091684 Free PMC article. Review.
Cited by
-
Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials.Exp Diabetes Res. 2011;2011:215764. doi: 10.1155/2011/215764. Epub 2011 Apr 26. Exp Diabetes Res. 2011. PMID: 21584276 Free PMC article.
-
Evolution of exenatide as a diabetes therapeutic.Curr Diabetes Rev. 2013 Mar 1;9(2):161-93. doi: 10.2174/1573399811309020007. Curr Diabetes Rev. 2013. PMID: 23256660 Free PMC article. Review.
-
GLP-1 Receptor Agonists for Type 2 Diabetes and Their Role in Primary Care: An Australian Perspective.Diabetes Ther. 2019 Aug;10(4):1205-1217. doi: 10.1007/s13300-019-0642-2. Epub 2019 Jun 10. Diabetes Ther. 2019. PMID: 31183762 Free PMC article. Review.
-
Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis.BMC Endocr Disord. 2010 Dec 9;10:20. doi: 10.1186/1472-6823-10-20. BMC Endocr Disord. 2010. PMID: 21143938 Free PMC article.
-
Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials.BMJ. 2012 Jan 10;344:d7771. doi: 10.1136/bmj.d7771. BMJ. 2012. PMID: 22236411 Free PMC article.
References
-
- Reaven GM: Banting Lecture 1988: Role of insulin resistance in human disease. Diabetes 1988; 37: 1595– 1607 - PubMed
-
- Wajchenberg BL: Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28: 187– 218 - PubMed
-
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2796– 2803 - PubMed
-
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292: E871– E883 - PubMed
-
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 - PubMed

