Conjunction of vocal production and perception regulates expression of the immediate early gene ZENK in a novel cortical region of songbirds
- PMID: 20107119
- PMCID: PMC2853286
- DOI: 10.1152/jn.00869.2009
Conjunction of vocal production and perception regulates expression of the immediate early gene ZENK in a novel cortical region of songbirds
Abstract
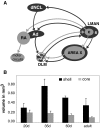
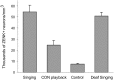
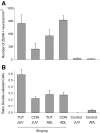
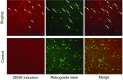
The cortical nucleus LMAN (lateral magnocellular nucleus of the anterior nidopallium) provides the output of a basal ganglia pathway that is necessary for acquisition of learned vocal behavior during development in songbirds. LMAN is composed of two subregions, a core and a surrounding shell, that give rise to independent pathways that traverse the forebrain in parallel. The LMAN(shell) pathway forms a recurrent loop that includes a cortical region, the dorsal region of the caudolateral nidopallium (dNCL), hitherto unknown to be involved with learned vocal behavior. Here we show that vocal production strongly induces the IEG product ZENK in dNCL of zebra finches. Hearing tutor song while singing is more effective at inducing expression in dNCL of juvenile birds during the auditory-motor integration stage of vocal learning than is hearing conspecific song. In contrast, hearing conspecific song is relatively more effective at inducing expression in adult birds, regardless of whether they are producing song. Furthermore, ZENK+ neurons in dNCL include projection neurons that are part of the LMAN(shell) recurrent loop and a high proportion of dNCL projection neurons express ZENK in singing juvenile birds that hear tutor song. Thus juvenile birds that are actively refining their vocal pattern to imitate a tutor song show high levels of ZENK induction in dNCL neurons when they are singing while hearing the song of their tutor and low levels when they hear a novel conspecific. This pattern indicates that dNCL is a novel brain region involved with vocal learning and that its function is developmentally regulated.
Figures


Similar articles
-
Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning.J Comp Neurol. 2000 May 1;420(2):244-60. J Comp Neurol. 2000. PMID: 10753310
-
Auditory experience refines cortico-basal ganglia inputs to motor cortex via remapping of single axons during vocal learning in zebra finches.J Neurophysiol. 2012 Feb;107(4):1142-56. doi: 10.1152/jn.00614.2011. Epub 2011 Dec 7. J Neurophysiol. 2012. PMID: 22157116 Free PMC article.
-
Silent synapses in a thalamo-cortical circuit necessary for song learning in zebra finches.J Neurophysiol. 2005 Dec;94(6):3698-707. doi: 10.1152/jn.00282.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107531
-
Auditory signal processing in communication: perception and performance of vocal sounds.Hear Res. 2013 Nov;305:144-55. doi: 10.1016/j.heares.2013.06.007. Epub 2013 Jul 1. Hear Res. 2013. PMID: 23827717 Free PMC article. Review.
-
Ethological concepts revisited: immediate early gene induction in response to sexual stimuli in birds.Brain Behav Evol. 2001 May;57(5):252-70. doi: 10.1159/000047244. Brain Behav Evol. 2001. PMID: 11641562 Review.
Cited by
-
Effect of vocal nerve section on song and ZENK protein expression in area X in adult male zebra finches.Neural Plast. 2012;2012:902510. doi: 10.1155/2012/902510. Epub 2012 Nov 26. Neural Plast. 2012. PMID: 23251821 Free PMC article.
-
Neural activity in cortico-basal ganglia circuits of juvenile songbirds encodes performance during goal-directed learning.Elife. 2017 Dec 19;6:e26973. doi: 10.7554/eLife.26973. Elife. 2017. PMID: 29256393 Free PMC article.
-
Neural representation of a target auditory memory in a cortico-basal ganglia pathway.J Neurosci. 2013 Sep 4;33(36):14475-88. doi: 10.1523/JNEUROSCI.0710-13.2013. J Neurosci. 2013. PMID: 24005299 Free PMC article.
-
Acute Aromatase Inhibition Impairs Neural and Behavioral Auditory Scene Analysis in Zebra Finches.eNeuro. 2024 Mar 22;11(3):ENEURO.0423-23.2024. doi: 10.1523/ENEURO.0423-23.2024. Print 2024 Mar. eNeuro. 2024. PMID: 38467426 Free PMC article.
-
Cortical inter-hemispheric circuits for multimodal vocal learning in songbirds.J Comp Neurol. 2017 Oct 15;525(15):3312-3340. doi: 10.1002/cne.24280. Epub 2017 Jul 25. J Comp Neurol. 2017. PMID: 28681379 Free PMC article.
References
-
- Bohner J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav 39: 369–374, 1990.
-
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci 7: 347–357, 2006. - PubMed
-
- Bottjer SW. Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann NY Acad Sci 1016: 395–415, 2004. - PubMed
-
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol 420: 244–260, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

