A low-affinity antagonist reveals saturation and desensitization in mature synapses in the auditory brain stem
- PMID: 20107122
- PMCID: PMC2853296
- DOI: 10.1152/jn.00751.2009
A low-affinity antagonist reveals saturation and desensitization in mature synapses in the auditory brain stem
Abstract
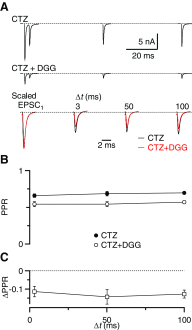
Postsynaptic receptor desensitization has been observed to contribute to depression in immature synapses. However, it is not clear whether desensitization persists and causes depression in mature synapses. We investigate this issue at the endbulb of Held, the synapse made by auditory nerve (AN) fibers onto bushy cells (BCs) of the anteroventral cochlear nucleus, where depression could influence the processing of sound information. Experiments using cyclothiazide (CTZ) have implicated desensitization in endbulbs from postnatal day 16 (P16) to P21 mice, but application of γ-D-glutamylglycine (DGG) did not reveal desensitization in endbulbs >P22. To reconcile these findings, we have studied the effects of both CTZ and DGG on endbulbs from P5 to P40 CBA/CaJ mice. In paired-pulse protocols, both CTZ and DGG reduced depression in all ages at intervals <10 ms, consistent with their effects preventing desensitization. However, DGG increased depression at intervals >20 ms, consistent with DGG's use to prevent saturation. DGG application revealed receptor saturation even under conditions of very low release probability. Preventing desensitization by CTZ occluded the effects of DGG on desensitization and revealed the effects of saturation at short intervals. We developed an approach to separate DGG's effect on saturation from its effect on desensitization, which showed that desensitization has an impact during bursts of auditory nerve activity. Dynamic-clamp experiments indicated that desensitization can reduce BC spike probability and increase latency and jitter. Thus desensitization may affect sound processing in the mature auditory system.
Figures







References
-
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159–170, 1999. - PubMed
-
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J Comp Neurol 160: 491–506, 1975. - PubMed
-
- Brawer JR, Morest DK, Kane EC. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol 155: 251–300, 1974. - PubMed
-
- Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron 33: 779–788, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

