Singing-related neural activity distinguishes four classes of putative striatal neurons in the songbird basal ganglia
- PMID: 20107125
- PMCID: PMC2853292
- DOI: 10.1152/jn.01038.2009
Singing-related neural activity distinguishes four classes of putative striatal neurons in the songbird basal ganglia
Abstract
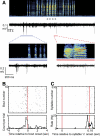
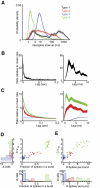
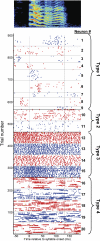
The striatum-the primary input nucleus of the basal ganglia-plays a major role in motor control and learning. Four main classes of striatal neuron are thought to be essential for normal striatal function: medium spiny neurons, fast-spiking interneurons, cholinergic tonically active neurons, and low-threshold spiking interneurons. However, the nature of the interaction of these neurons during behavior is poorly understood. The songbird area X is a specialized striato-pallidal basal ganglia nucleus that contains two pallidal cell types as well as the same four cell types found in the mammalian striatum. We recorded 185 single units in Area X of singing juvenile birds and, based on singing-related firing patterns and spike waveforms, find six distinct cell classes--two classes of putative pallidal neuron that exhibited a high spontaneous firing rate (> 60 Hz), and four cell classes that exhibited low spontaneous firing rates characteristic of striatal neurons. In this study, we examine in detail the four putative striatal cell classes. Type-1 neurons were the most frequently encountered and exhibited sparse temporally precise singing-related activity. Type-2 neurons were distinguished by their narrow spike waveforms and exhibited brief, high-frequency bursts during singing. Type-3 neurons were tonically active and did not burst, whereas type-4 neurons were inactive outside of singing and during singing generated long high-frequency bursts that could reach firing rates over 1 kHz. Based on comparison to the mammalian literature, we suggest that these four putative striatal cell classes correspond, respectively, to the medium spiny neurons, fast-spiking interneurons, tonically active neurons, and low-threshold spiking interneurons that are known to reside in area X.
Figures





Similar articles
-
Singing-related neural activity distinguishes two putative pallidal cell types in the songbird basal ganglia: comparison to the primate internal and external pallidal segments.J Neurosci. 2010 May 19;30(20):7088-98. doi: 10.1523/JNEUROSCI.0168-10.2010. J Neurosci. 2010. PMID: 20484651 Free PMC article.
-
Social context differentially modulates activity of two interneuron populations in an avian basal ganglia nucleus.J Neurophysiol. 2016 Dec 1;116(6):2831-2840. doi: 10.1152/jn.00622.2016. Epub 2016 Sep 14. J Neurophysiol. 2016. PMID: 27628208 Free PMC article.
-
Origins of basal ganglia output signals in singing juvenile birds.J Neurophysiol. 2015 Feb 1;113(3):843-55. doi: 10.1152/jn.00635.2014. Epub 2014 Nov 12. J Neurophysiol. 2015. PMID: 25392171 Free PMC article.
-
Spike-timing dependent plasticity in striatal interneurons.Neuropharmacology. 2011 Apr;60(5):780-8. doi: 10.1016/j.neuropharm.2011.01.023. Epub 2011 Jan 22. Neuropharmacology. 2011. PMID: 21262240 Review.
-
Optogenetic insights into striatal function and behavior.Behav Brain Res. 2013 Oct 15;255:44-54. doi: 10.1016/j.bbr.2013.04.018. Epub 2013 Apr 28. Behav Brain Res. 2013. PMID: 23628212 Review.
Cited by
-
Integration of cortical and pallidal inputs in the basal ganglia-recipient thalamus of singing birds.J Neurophysiol. 2012 Sep;108(5):1403-29. doi: 10.1152/jn.00056.2012. Epub 2012 Jun 6. J Neurophysiol. 2012. PMID: 22673333 Free PMC article.
-
Maturation, Behavioral Activation, and Connectivity of Adult-Born Medium Spiny Neurons in a Striatal Song Nucleus.Front Neurosci. 2017 Jun 7;11:323. doi: 10.3389/fnins.2017.00323. eCollection 2017. Front Neurosci. 2017. PMID: 28638318 Free PMC article.
-
A hypothesis for basal ganglia-dependent reinforcement learning in the songbird.Neuroscience. 2011 Dec 15;198:152-70. doi: 10.1016/j.neuroscience.2011.09.069. Epub 2011 Oct 13. Neuroscience. 2011. PMID: 22015923 Free PMC article. Review.
-
Design and assembly of an ultra-light motorized microdrive for chronic neural recordings in small animals.J Vis Exp. 2012 Nov 8;(69):4314. doi: 10.3791/4314. J Vis Exp. 2012. PMID: 23169237 Free PMC article.
-
Endocannabinoids mediate synaptic plasticity at glutamatergic synapses on spiny neurons within a basal ganglia nucleus necessary for song learning.J Neurophysiol. 2011 Mar;105(3):1159-69. doi: 10.1152/jn.00676.2010. Epub 2010 Dec 22. J Neurophysiol. 2011. PMID: 21177997 Free PMC article.
References
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol 73: 1234–1252, 1995 - PubMed
-
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci 30: 299–306, 2007 - PubMed
-
- Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res 84: 672–675, 1991 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

