Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation
- PMID: 20107188
- PMCID: PMC4256430
- DOI: 10.4049/jimmunol.0903526
Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation
Erratum in
- J Immunol. 2011 Feb 15;186(4):2684-5
Abstract
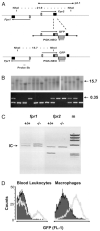
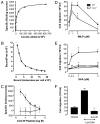
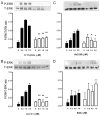
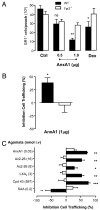
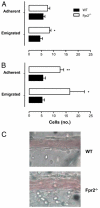
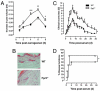
The human formyl-peptide receptor (FPR)-2 is a G protein-coupled receptor that transduces signals from lipoxin A(4), annexin A1, and serum amyloid A (SAA) to regulate inflammation. In this study, we report the creation of a novel mouse colony in which the murine FprL1 FPR2 homologue, Fpr2, has been deleted and describe its use to explore the biology of this receptor. Deletion of murine fpr2 was verified by Southern blot analysis and PCR, and the functional absence of the G protein-coupled receptor was confirmed by radioligand binding assays. In vitro, Fpr2(-/-) macrophages had a diminished response to formyl-Met-Leu-Phe itself and did not respond to SAA-induced chemotaxis. ERK phosphorylation triggered by SAA was unchanged, but that induced by the annexin A1-derived peptide Ac2-26 or other Fpr2 ligands, such as W-peptide and compound 43, was attenuated markedly. In vivo, the antimigratory properties of compound 43, lipoxin A(4), annexin A1, and dexamethasone were reduced notably in Fpr2(-/-) mice compared with those in wild-type littermates. In contrast, SAA stimulated neutrophil recruitment, but the promigratory effect was lost following Fpr2 deletion. Inflammation was more marked in Fpr2(-/-) mice, with a pronounced increase in cell adherence and emigration in the mesenteric microcirculation after an ischemia-reperfusion insult and an augmented acute response to carrageenan-induced paw edema, compared with that in wild-type controls. Finally, Fpr2(-/-) mice exhibited higher sensitivity to arthrogenic serum and were completely unable to resolve this chronic pathology. We conclude that Fpr2 is an anti-inflammatory receptor that serves varied regulatory functions during the host defense response. These data support the development of Fpr2 agonists as novel anti-inflammatory therapeutics.
Figures
References
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
-
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. - PubMed
-
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. - PubMed
-
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

