Donor-host involvement in immature rat testis xenografting into nude mouse hosts
- PMID: 20107205
- PMCID: PMC2857632
- DOI: 10.1095/biolreprod.109.082073
Donor-host involvement in immature rat testis xenografting into nude mouse hosts
Abstract
Immature testicular tissue of a wide variety of mammalian species continues growth and maturation when ectopically grafted under the dorsal skin of adult nude mouse recipients. Tissues from most donor species fully mature, exhibiting complete spermatogenesis within months. The connection to the recipient's vascular system is mandatory for graft development, and failure of vascularization leads to necrosis in the grafted tissue. In the present study, we analyze to what extent 1) the xenografted immature donor tissue and 2) the recipient's cells and tissues contribute to the functional recovery of a "testicular xenograft." We address whether recipient cells migrate into the testicular parenchyma and whether the circulatory connection between the donor testicular tissue and the recipient is established by ingrowing host or outgrowing donor blood vessels. Although this issue has been repeatedly discussed in previous xenografting studies, so far it has not been possible to unequivocally distinguish between donor and recipient tissues and thus to identify the mechanisms by which the circulatory connection is established. To facilitate the distinction of donor and recipient tissues, herein we used immature green fluorescent protein-positive rat testes as donor tissues and adult nude mice as graft recipients. At the time of graft recovery, donor tissues could be easily identified by the GFP expression in these tissues, allowing us to distinguish donor- and recipient-derived blood vessels. We conclude that the circulatory connection between graft and host is established by a combination of outgrowing small capillaries from the donor tissue and formation of larger vessels by the host, which connect the graft to subcutaneous blood vessels.
Figures
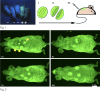
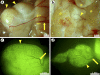
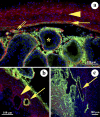


Similar articles
-
Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system.Reproduction. 2014 Nov;148(5):R71-84. doi: 10.1530/REP-14-0249. Epub 2014 Aug 22. Reproduction. 2014. PMID: 25150043 Free PMC article. Review.
-
The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice.Theriogenology. 2010 Mar 1;73(4):512-22. doi: 10.1016/j.theriogenology.2009.09.035. Epub 2009 Dec 4. Theriogenology. 2010. PMID: 19962749
-
Maturation of testicular tissue from infant monkeys after xenografting into mice.Endocrinology. 2008 Oct;149(10):5288-96. doi: 10.1210/en.2008-0311. Epub 2008 Jun 19. Endocrinology. 2008. PMID: 18566126 Free PMC article.
-
Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age.Biol Reprod. 2005 Feb;72(2):358-64. doi: 10.1095/biolreprod.104.030783. Epub 2004 Oct 6. Biol Reprod. 2005. PMID: 15470000
-
Germ cell transplantation and testis tissue xenografting in domestic animals.Anim Reprod Sci. 2005 Oct;89(1-4):137-45. doi: 10.1016/j.anireprosci.2005.06.020. Anim Reprod Sci. 2005. PMID: 16055282 Review.
Cited by
-
Fertility Preservation and Restoration Options for Pre-Pubertal Male Cancer Patients: Current Approaches.Front Endocrinol (Lausanne). 2022 Jun 16;13:877537. doi: 10.3389/fendo.2022.877537. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35784573 Free PMC article. Review.
-
Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments.Int J Mol Sci. 2018 Jan 18;19(1):286. doi: 10.3390/ijms19010286. Int J Mol Sci. 2018. PMID: 29346308 Free PMC article. Review.
-
Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system.Reproduction. 2014 Nov;148(5):R71-84. doi: 10.1530/REP-14-0249. Epub 2014 Aug 22. Reproduction. 2014. PMID: 25150043 Free PMC article. Review.
-
Significant Benefits of Nanoparticles Containing a Necrosis Inhibitor on Mice Testicular Tissue Autografts Outcomes.Int J Mol Sci. 2019 Nov 20;20(23):5833. doi: 10.3390/ijms20235833. Int J Mol Sci. 2019. PMID: 31757040 Free PMC article.
-
Differentiation of Testis Xenografts in the Prepubertal Marmoset Depends on the Sex and Status of the Mouse Host.Front Endocrinol (Lausanne). 2018 Aug 29;9:467. doi: 10.3389/fendo.2018.00467. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30210448 Free PMC article.
References
-
- Schlatt S, Kim SS, Gosden R.Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 2002; 124: 339–346. - PubMed
-
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I.Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 2004; 70: 1500–1503. - PubMed
-
- Jahnukainen K, Ehmcke J, Schlatt S.Testicular xenografts: a novel approach to study cytotoxic damage in juvenile primate testis. Cancer Res 2006; 66: 3813–3818. - PubMed
-
- Ehmcke J, Gassei K, Schlatt S.Ectopic testicular xenografts from newborn hamsters (Phodopus sungorus) show better spermatogenic activity in aged compared with young recipients. J Exp Zool A Ecol Genet Physiol 2008; 309: 278–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

