Vaccine-acquired rotavirus in infants with severe combined immunodeficiency
- PMID: 20107217
- PMCID: PMC4103739
- DOI: 10.1056/NEJMoa0904485
Vaccine-acquired rotavirus in infants with severe combined immunodeficiency
Abstract
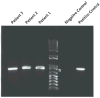
Live pentavalent human-bovine reassortant rotavirus vaccine is recommended in the United States for routine immunization of infants. We describe three infants, two with failure to thrive, who had dehydration and diarrhea within 1 month after their first or second rotavirus immunization and subsequently received a diagnosis of severe combined immunodeficiency. Rotavirus was detected, by means of reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay, in stool specimens obtained from all three infants, and gene-sequence analysis revealed the presence of vaccine rotavirus. These infections raise concerns regarding the safety of rotavirus vaccine in severely immunocompromised patients.
2010 Massachusetts Medical Society
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(RR-2):1–25. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. - PubMed
-
- Pariyaprasert W, Pacharn P, Visitsunthorn N, et al. Successful treatment of disseminated BCG infection in a SCID patient with granulocyte colony stimulating factor. Asian Pac J Allergy Immunol. 2008;26:71–5. - PubMed
-
- Culic S, Kuzmic I, Culic V, et al. Disseminated BCG infection resembling Langerhans cell histiocytosis in an infant with severe combined immunodeficiency: a case report. Pediatr Hematol Oncol. 2004;21:563–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
