Direct conversion of fibroblasts to functional neurons by defined factors
- PMID: 20107439
- PMCID: PMC2829121
- DOI: 10.1038/nature08797
Direct conversion of fibroblasts to functional neurons by defined factors
Abstract
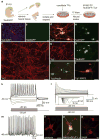
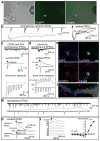
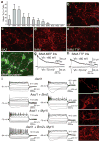
Cellular differentiation and lineage commitment are considered to be robust and irreversible processes during development. Recent work has shown that mouse and human fibroblasts can be reprogrammed to a pluripotent state with a combination of four transcription factors. This raised the question of whether transcription factors could directly induce other defined somatic cell fates, and not only an undifferentiated state. We hypothesized that combinatorial expression of neural-lineage-specific transcription factors could directly convert fibroblasts into neurons. Starting from a pool of nineteen candidate genes, we identified a combination of only three factors, Ascl1, Brn2 (also called Pou3f2) and Myt1l, that suffice to rapidly and efficiently convert mouse embryonic and postnatal fibroblasts into functional neurons in vitro. These induced neuronal (iN) cells express multiple neuron-specific proteins, generate action potentials and form functional synapses. Generation of iN cells from non-neural lineages could have important implications for studies of neural development, neurological disease modelling and regenerative medicine.
Figures


Comment in
-
Regenerative medicine: Cell reprogramming gets direct.Nature. 2010 Feb 25;463(7284):1031-2. doi: 10.1038/4631031a. Nature. 2010. PMID: 20182502 No abstract available.
-
What are the limits to cell plasticity?Cell Res. 2010 May;20(5):502-3. doi: 10.1038/cr.2010.59. Cell Res. 2010. PMID: 20436508 No abstract available.
References
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. - PubMed
-
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–81. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

