Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naïve adults
- PMID: 20107498
- PMCID: PMC2809736
- DOI: 10.1371/journal.pone.0008787
Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naïve adults
Abstract
Background: Merozoite surface protein 1(42) (MSP1(42)) is a leading blood stage malaria vaccine candidate. In order to induce immune responses that cover the major antigenic polymorphisms, FVO and 3D7 recombinant proteins of MSP1(42) were mixed (MSP1(42)-C1). To improve the level of antibody response, MSP1(42)-C1 was formulated with Alhydrogel plus the novel adjuvant CPG 7909.
Methods: A Phase 1 clinical trial was conducted in healthy malaria-naïve adults at the Center for Immunization Research in Washington, D.C., to evaluate the safety and immunogenicity of MSP1(42)-C1/Alhydrogel +/- CPG 7909. Sixty volunteers were enrolled in dose escalating cohorts and randomized to receive three vaccinations of either 40 or 160 microg protein adsorbed to Alhydrogel +/- 560 microg CPG 7909 at 0, 1 and 2 months.
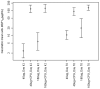
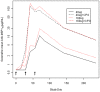
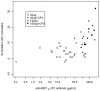
Results: Vaccinations were well tolerated, with only one related adverse event graded as severe (Grade 3 injection site erythema) and all other vaccine related adverse events graded as either mild or moderate. Local adverse events were more frequent and severe in the groups receiving CPG. The addition of CPG enhanced anti-MSP1(42) antibody responses following vaccination by up to 49-fold two weeks after second immunization and 8-fold two weeks after the third immunization when compared to MSP1(42)-C1/Alhydrogel alone (p<0.0001). After the third immunization, functionality of the antibody was tested by an in vitro growth inhibition assay. Inhibition was a function of antibody titer, with an average of 3% (range -2 to 10%) in the non CPG groups versus 14% (3 to 32%) in the CPG groups.
Conclusion/significance: The favorable safety profile and high antibody responses induced with MSP1(42)-C1/Alhydrogel + CPG 7909 are encouraging. MSP1(42)-C1/Alhydrogel is being combined with other blood stage antigens and will be taken forward in a formulation adjuvanted with CPG 7909.
Trial registration: ClinicalTrials.gov Identifier: NCT00320658.
Conflict of interest statement
Figures
Similar articles
-
Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria.PLoS One. 2008 Aug 13;3(8):e2940. doi: 10.1371/journal.pone.0002940. PLoS One. 2008. PMID: 18698359 Free PMC article. Clinical Trial.
-
Phase 1 study in malaria naïve adults of BSAM2/Alhydrogel®+CPG 7909, a blood stage vaccine against P. falciparum malaria.PLoS One. 2012;7(10):e46094. doi: 10.1371/journal.pone.0046094. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056238 Free PMC article. Clinical Trial.
-
Results from tandem Phase 1 studies evaluating the safety, reactogenicity and immunogenicity of the vaccine candidate antigen Plasmodium falciparum FVO merozoite surface protein-1 (MSP1(42)) administered intramuscularly with adjuvant system AS01.Malar J. 2013 Jan 23;12:29. doi: 10.1186/1475-2875-12-29. Malar J. 2013. PMID: 23342996 Free PMC article. Clinical Trial.
-
Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide.Vaccine. 2006 Mar 24;24(14):2497-505. doi: 10.1016/j.vaccine.2005.12.034. Epub 2006 Jan 4. Vaccine. 2006. PMID: 16434128
-
Phase 1 study of two merozoite surface protein 1 (MSP1(42)) vaccines for Plasmodium falciparum malaria.PLoS Clin Trials. 2007;2(4):e12. doi: 10.1371/journal.pctr.0020012. Epub 2007 Apr 6. PLoS Clin Trials. 2007. PMID: 17415408 Free PMC article.
Cited by
-
A vaccine based on the yeast-expressed receptor-binding domain (RBD) elicits broad immune responses against SARS-CoV-2 variants.Front Immunol. 2022 Nov 9;13:1011484. doi: 10.3389/fimmu.2022.1011484. eCollection 2022. Front Immunol. 2022. PMID: 36439096 Free PMC article.
-
Blood stage merozoite surface protein conjugated to nanoparticles induce potent parasite inhibitory antibodies.Vaccine. 2011 Nov 8;29(48):8898-908. doi: 10.1016/j.vaccine.2011.09.070. Epub 2011 Sep 28. Vaccine. 2011. PMID: 21963870 Free PMC article.
-
Recent advances in recombinant protein-based malaria vaccines.Vaccine. 2015 Dec 22;33(52):7433-43. doi: 10.1016/j.vaccine.2015.09.093. Epub 2015 Oct 11. Vaccine. 2015. PMID: 26458807 Free PMC article. Review.
-
Plasmodium knowlesi malaria: current research perspectives.Infect Drug Resist. 2018 Aug 10;11:1145-1155. doi: 10.2147/IDR.S148664. eCollection 2018. Infect Drug Resist. 2018. PMID: 30127631 Free PMC article. Review.
-
TLR-based immune adjuvants.Vaccine. 2011 Apr 12;29(17):3341-55. doi: 10.1016/j.vaccine.2010.08.002. Epub 2010 Aug 14. Vaccine. 2011. PMID: 20713100 Free PMC article. Review.
References
-
- WHO. (2008) World Malaria Report, 2008. Available: http://apps.who.int/malaria/wmr2008/malaria2008.pdf.
-
- Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. - PubMed
-
- Ballou WR. The development of the RTS,S malaria vaccine candidate: challenges and lessons, Parasit Immunol., 2009;31:492–500. - PubMed
-
- Cohen S, McGregor IA, Carrington Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. - PubMed
-
- Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45(3):297–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

