Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials
- PMID: 20107502
- PMCID: PMC2809740
- DOI: 10.1371/journal.pone.0008781
Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials
Abstract
Background: The pharmacokinetics and pharmacodynamics of vaginal microbicides are typically assessed among sexually abstinent women. However, the physical act of sex may modulate gel distribution, and preclinical studies demonstrate seminal plasma interferes with the antiviral activity of several microbicides. This study compared the biological activity and concentration of PRO 2000 in cervicovaginal lavage (CVL) collected in the absence or following coitus.
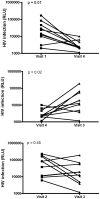
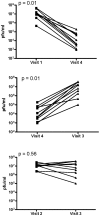
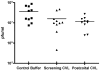
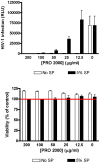
Methods: CVL samples were collected from ten heterosexual couples at baseline, after sex, after a single dose of 0.5% PRO 2000 gel and sex, and after gel application without sex. The impact of CVL on HIV-1 infection of TZM-bl cells and HSV-2 infection of CaSki cells was monitored by luciferase and plaque assay, respectively. PRO 2000 concentrations were measured by fluorescence.
Results: CVL collected after PRO 2000 application significantly inhibited HIV-1 and HSV-2 (p = 0.01). However, the antiviral activity was reduced following sex and no significant protective effect was observed in postcoital CVL obtained in the presence compared to the absence of PRO 2000 for HIV (p = 0.45) or HSV-2 (p = 0.56). Less PRO 2000 was recovered in postcoital CVL, which, in conjunction with interference by seminal plasma, may have contributed to lower antiviral activity.
Conclusions: Postcoital responses to PRO 2000 differ from precoital measures and the results obtained may provide insights into the clinical trial findings in which there was no significant protection against HIV-1 or HSV-2. Postcoital studies should be incorporated into clinical studies before embarking on large-scale efficacy trials.
Conflict of interest statement
Figures

References
-
- Karim SA, Coletti A, Richardson B, Ramjee G, Hoffman I, et al. Safety and Effectiveness of Vaginal Microbicides BufferGel and 0.5% PRO 2000/5 Gel for the Prevention of HIV Infection in Women: Results of the HPTN 035 Trial 16th Conference on Retroviruses and Opportunistic Infections. 2009 Montreal, Canada.
-
- Microbicide Development Programme. 2009. HIV ‘prevention’ gel PRO 2000 proven ineffective. Available: http://www.mdp.mrc.ac.uk/. Accessed 2009 Dec 14.
-
- Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. - PubMed
-
- Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

